

November 24, 2021 Annual Meeting of Stockholders CytoDyn’s PRESENTATION The pursuit of precision medicine Humanized Monoclonal Antibody Exhibit 99.1

This presentation contains certain forward-looking statements that involve risks, uncertainties and assumptions that are difficult to predict. Words and expressions reflecting optimism, satisfaction or disappointment with current prospects, as well as words such as "believes," "hopes," "intends," "estimates," "expects," "projects," "plans," "anticipates" and variations thereof, or the use of future tense, identify forward-looking statements, but their absence does not mean that a statement is not forward-looking. Forward-looking statements specifically include statements about leronlimab, its ability to provide positive health outcomes, the possible results of clinical trials, studies or other programs or ability to continue those programs, the ability to obtain regulatory approval for commercial sales, and the market for actual commercial sales. The Company’s forward-looking statements are not guarantees of performance, and actual results could vary materially from those contained in or expressed by such statements due to risks and uncertainties including: (i) the regulatory determinations of leronlimab’s efficacy to treat human immunodeficiency virus (“HIV”) patients with multiple resistance to current standard of care, COVID-19 patients, and metastatic Triple-Negative Breast Cancer (“mTNBC”), among other indications, by the U.S. Food and Drug Administration and various drug regulatory agencies in other countries; (ii) the Company’s ability to raise additional capital to fund its operations; (iii) the Company’s ability to meet its debt obligations; (iv) the Company’s ability to enter into partnership or licensing arrangements with third-parties; (v) the Company’s ability to identify patients to enroll in its clinical trials in a timely fashion; (vi) the Company’s ability to achieve approval of a marketable product; (vii) the design, implementation and conduct of the Company’s clinical trials; (viii) the results of the Company’s clinical trials, including the possibility of unfavorable clinical trial results; (ix) the market for, and marketability of, any product that is approved; (x) the existence or development of vaccines, drugs, or other treatments that are viewed by medical professionals or patients as superior to the Company’s products; (xi) regulatory initiatives, compliance with governmental regulations and the regulatory approval process; (xii) legal proceedings, investigations or inquiries affecting the Company or its products; (xiii) general economic and business conditions; (xiv) changes in foreign, political, and social conditions; (xv) stockholder actions or proposals with regard to the Company, its management, or its board of directors; and (xvi) various other matters, many of which are beyond the Company’s control. The Company urges investors to consider specifically the various risk factors identified in its most recent Form 10-K, and any risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the Securities and Exchange Commission. Except as required by law, the Company does not undertake any responsibility to update any forward-looking statements to take into account events or circumstances that occur after the date of this presentation. Forward-Looking Information and Statements
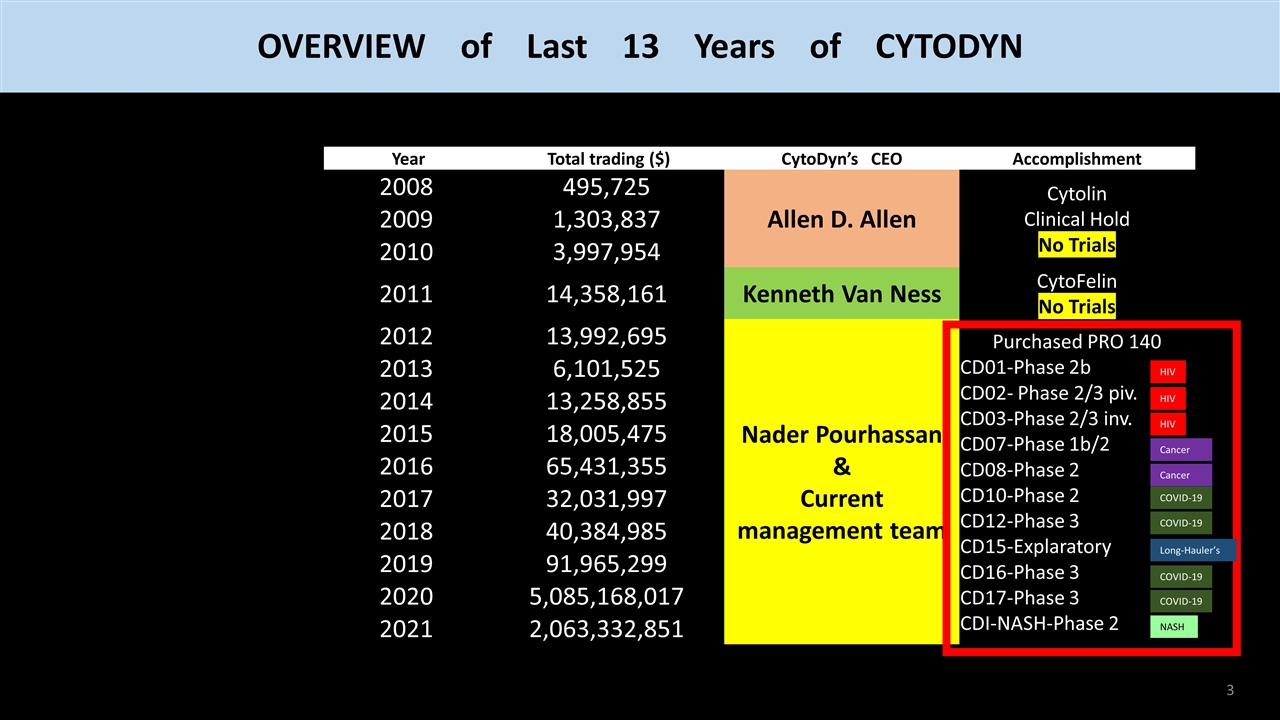
OVERVIEW of Last 13 Years of CYTODYN Year Total trading ($) CytoDyn’s CEO Accomplishment 2008 495,725 Allen D. Allen Cytolin Clinical Hold No Trials 2009 1,303,837 2010 3,997,954 2011 14,358,161 Kenneth Van Ness CytoFelin No Trials 2012 13,992,695 Nader Pourhassan Purchased PRO 140 CD01-Phase 2b CD02- Phase 2/3 piv. CD03-Phase 2/3 inv. CD10-Phase 2 CD12-Phase 3 CD15-Explaratory CD16-Phase 3 CD17-Phase 3 CD-NASH-Phase 2 2013 6,101,525 2014 13,258,855 2015 18,005,475 2016 65,431,355 2017 32,031,997 2018 40,384,985 2019 91,965,299 2020 5,085,168,017 2021 2,063,332,851 2022 ???? ???? Year Total trading ($) CytoDyn’s CEO Accomplishment 2008 495,725 Allen D. Allen Cytolin Clinical Hold No Trials 2009 1,303,837 2010 3,997,954 2011 14,358,161 Kenneth Van Ness CytoFelin No Trials 2012 13,992,695 Nader Pourhassan Purchased PRO 140 CD01-Phase 2b CD02- Phase 2/3 piv. CD03-Phase 2/3 inv. CD10-Phase 2 CD12-Phase 3 CD15-Explaratory CD16-Phase 3 CD17-Phase 3 CD-NASH-Phase 2 2013 6,101,525 2014 13,258,855 2015 18,005,475 2016 65,431,355 2017 32,031,997 2018 40,384,985 2019 91,965,299 2020 5,085,168,017 2021 2,063,332,851 2022 ???? ???? Year Total trading ($) CytoDyn’s CEO Accomplishment 2008 495,725 Allen D. Allen Cytolin Clinical Hold No Trials 2009 1,303,837 2010 3,997,954 2011 14,358,161 Kenneth Van Ness CytoFelin No Trials 2012 13,992,695 Nader Pourhassan & Current management team Purchased PRO 140 CD01-Phase 2b CD02- Phase 2/3 piv. CD03-Phase 2/3 inv. CD10-Phase 2 CD12-Phase 3 CD15-Explaratory CD16-Phase 3 CD17-Phase 3 CD-NASH-Phase 2 2013 6,101,525 2014 13,258,855 2015 18,005,475 2016 65,431,355 2017 32,031,997 2018 40,384,985 2019 91,965,299 2020 5,085,168,017 2021 2,063,332,851 2022 ???? ???? Year Total trading ($) CytoDyn’s CEO Accomplishment 2008 495,725 Allen D. Allen Cytolin Clinical Hold No Trials 2009 1,303,837 2010 3,997,954 2011 14,358,161 Kenneth Van Ness CytoFelin No Trials 2012 13,992,695 Nader Pourhassan & Current management team Purchased PRO 140 CD01-Phase 2b CD02- Phase 2/3 piv. CD03-Phase 2/3 inv. CD10-Phase 2 CD12-Phase 3 CD15-Explaratory CD16-Phase 3 CD17-Phase 3 CD-NASH-Phase 2 2013 6,101,525 2014 13,258,855 2015 18,005,475 2016 65,431,355 2017 32,031,997 2018 40,384,985 2019 91,965,299 2020 5,085,168,017 2021 2,063,332,851 2022 ???? ???? More than 10,000 times higher Year Total trading ($) CytoDyn’s CEO Accomplishment 2008 495,725 Allen D. Allen Cytolin Clinical Hold No Trials 2009 1,303,837 2010 3,997,954 2011 14,358,161 Kenneth Van Ness CytoFelin No Trials 2012 13,992,695 Nader Pourhassan & Current management team Purchased PRO 140 CD01-Phase 2b CD02- Phase 2/3 piv. CD03-Phase 2/3 inv. CD07-Phase 1b/2 CD08-Phase 2 CD10-Phase 2 CD12-Phase 3 CD15-Explaratory CD16-Phase 3 CD17-Phase 3 CDI-NASH-Phase 2 2013 6,101,525 2014 13,258,855 2015 18,005,475 2016 65,431,355 2017 32,031,997 2018 40,384,985 2019 91,965,299 2020 5,085,168,017 2021 2,063,332,851 2022 ???? ???? HIV HIV HIV Cancer Cancer COVID-19 COVID-19 Long-Hauler’s COVID-19 COVID-19 NASH
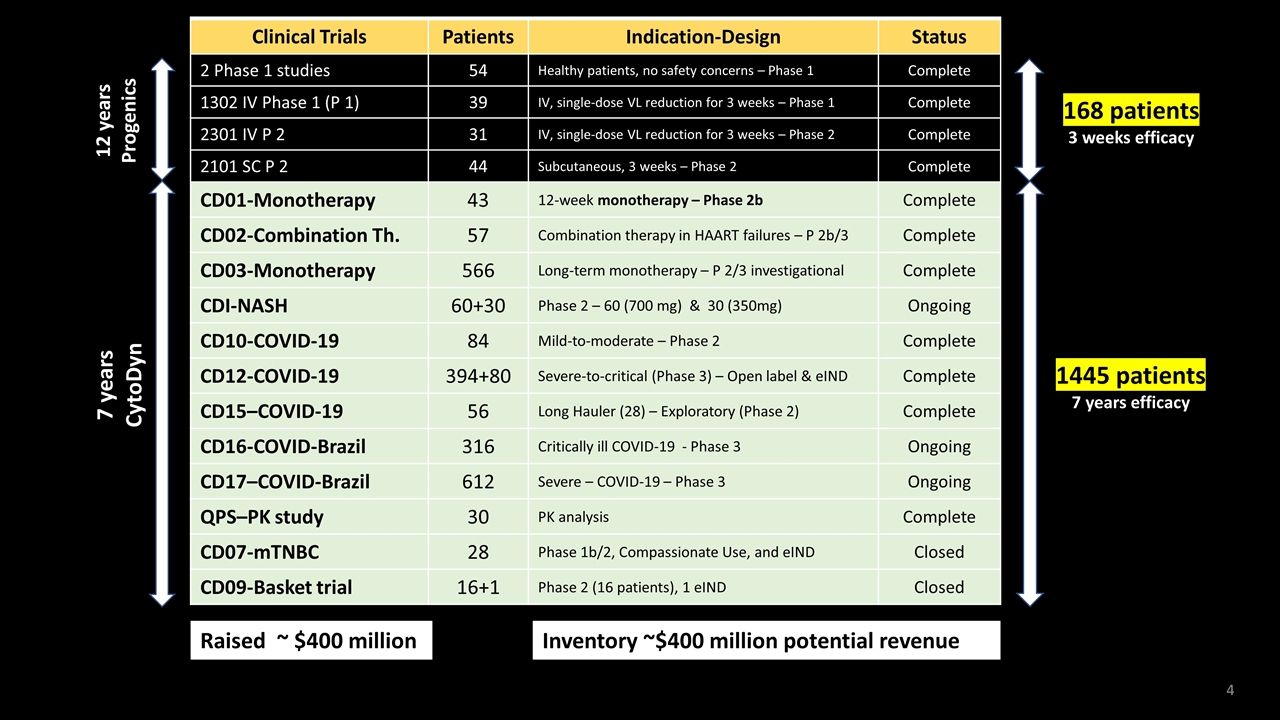
Clinical Trials Patients Indication-Design Status 2 Phase 1 studies 54 Healthy patients, no safety concerns – Phase 1 Complete 1302 IV Phase 1 (P 1) 39 IV, single-dose VL reduction for 3 weeks – Phase 1 Complete 2301 IV P 2 31 IV, single-dose VL reduction for 3 weeks – Phase 2 Complete 2101 SC P 2 44 Subcutaneous, 3 weeks – Phase 2 Complete CD01-Monotherapy 43 12-week monotherapy – Phase 2b Complete CD02-Combination Th. 57 Combination therapy in HAART failures – P 2b/3 Complete CD03-Monotherapy 566 Long-term monotherapy – P 2/3 investigational Complete CDI-NASH 60+30 Phase 2 – 60 (700 mg) & 30 (350mg) Ongoing CD10-COVID-19 84 Mild-to-moderate – Phase 2 Complete CD12-COVID-19 394+80 Severe-to-critical (Phase 3) – Open label & eIND Complete CD15–COVID-19 56 Long Hauler (28) – Exploratory (Phase 2) Complete CD16-COVID-Brazil 316 Critically ill COVID-19 - Phase 3 Ongoing CD17–COVID-Brazil 612 Severe – COVID-19 – Phase 3 Ongoing QPS–PK study 30 PK analysis Complete CD07-mTNBC 28 Phase 1b/2, Compassionate Use, and eIND Closed CD09-Basket trial 16+1 Phase 2 (16 patients), 1 eIND Closed 168 patients 3 weeks efficacy 12 years Progenics Clinical Trials Patients Indication-Design Status 2 Phase 1 studies 54 Healthy patients, no safety concerns – Phase 1 Complete 1302 IV Phase 1 (P 1) 39 IV, single-dose VL reduction for 3 weeks – Phase 1 Complete 2301 IV P 2 31 IV, single-dose VL reduction for 3 weeks – Phase 2 Complete 2101 SC P 2 44 Subcutaneous, 3 weeks – Phase 2 Complete CD01-Monotherapy 43 12-week monotherapy – Phase 2b Complete CD02-Combination Th. 57 Combination therapy in HAART failures – P 2b/3 Complete CD03-Monotherapy 566 Long-term monotherapy – P 2/3 investigational Complete CDI-NASH 60+30 Phase 2 – 60 (700 mg) & 30 (350mg) Ongoing CD10-COVID-19 84 Mild-to-moderate – Phase 2 Complete CD12-COVID-19 394+80 Severe-to-critical (Phase 3) – Open label & eIND Complete CD15–COVID-19 56 Long Hauler (28) – Exploratory (Phase 2) Complete CD16-COVID-Brazil 316 Critically ill COVID-19 - Phase 3 Ongoing CD17–COVID-Brazil 612 Severe – COVID-19 – Phase 3 Ongoing QPS–PK study 30 PK analysis Complete CD07-mTNBC 28 Phase 1b/2, Compassionate Use, and eIND Closed CD09-Basket trial 16+1 Phase 2 (16 patients), 1 eIND Closed 7 years CytoDyn 1445 patients 7 years efficacy Raised ~ $400 million Inventory ~$400 million potential revenue
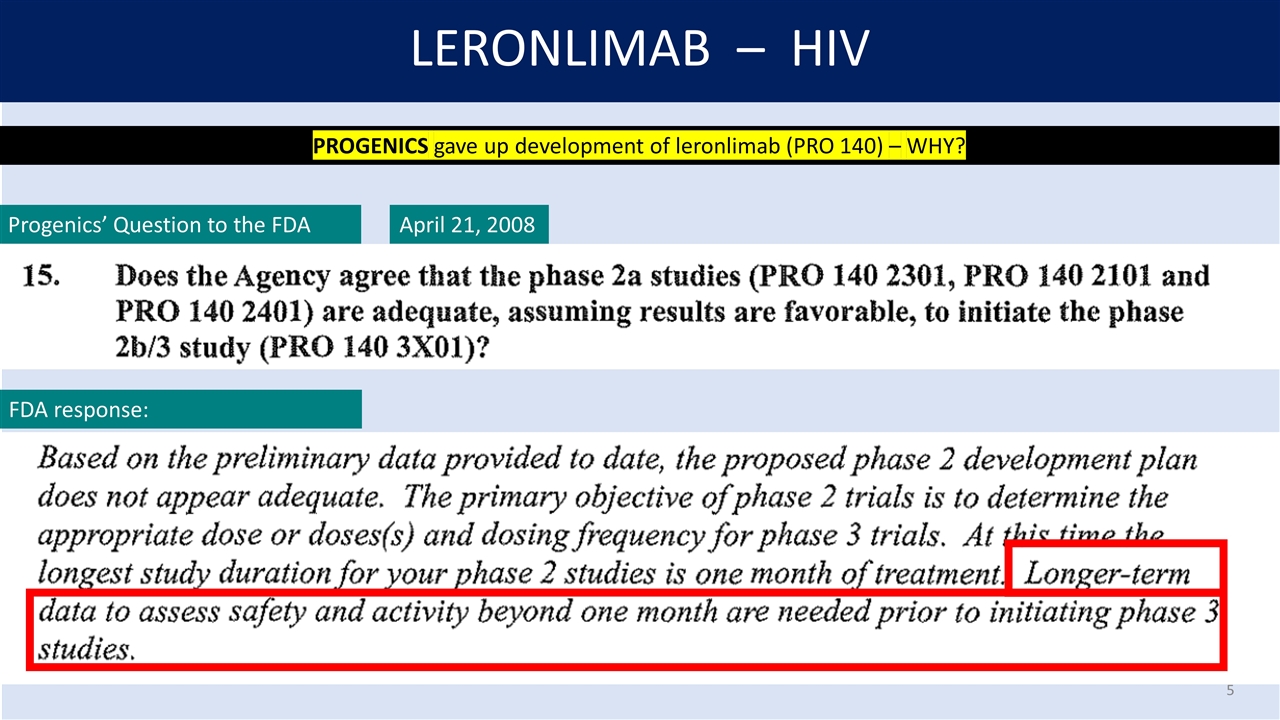
LERONLIMAB – HIV April 21, 2008 PROGENICS gave up development of leronlimab (PRO 140) – WHY? Progenics’ Question to the FDA FDA response:
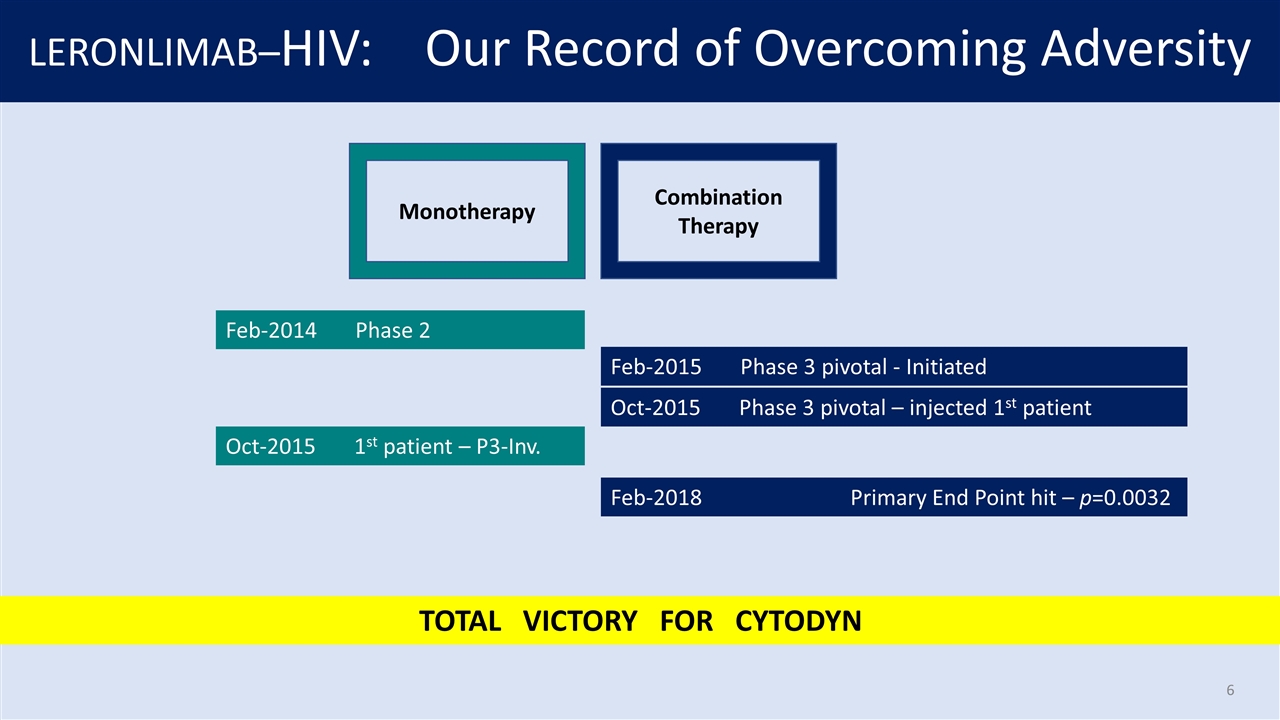
LERONLIMAB–HIV: Our Record of Overcoming Adversity Monotherapy Combination Therapy Feb-2014 Phase 2 Feb-2015 Phase 3 pivotal - Initiated Oct-2015 Phase 3 pivotal – injected 1st patient Oct-2015 1st patient – P3-Inv. Feb-2018 Primary End Point hit – p=0.0032 TOTAL VICTORY FOR CYTODYN
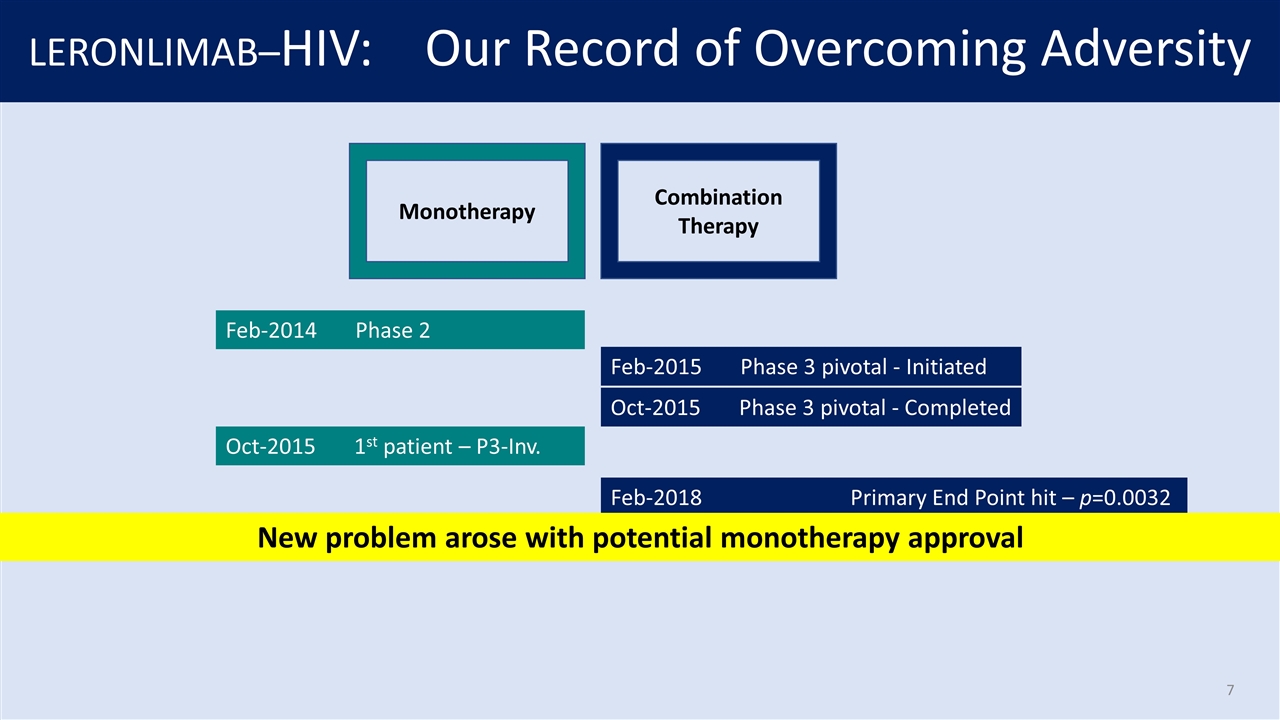
LERONLIMAB–HIV: Our Record of Overcoming Adversity Monotherapy Combination Therapy Feb-2014 Phase 2 Feb-2015 Phase 3 pivotal - Initiated Oct-2015 Phase 3 pivotal - Completed Oct-2015 1st patient – P3-Inv. Feb-2018 Primary End Point hit – p=0.0032 New problem arose with potential monotherapy approval
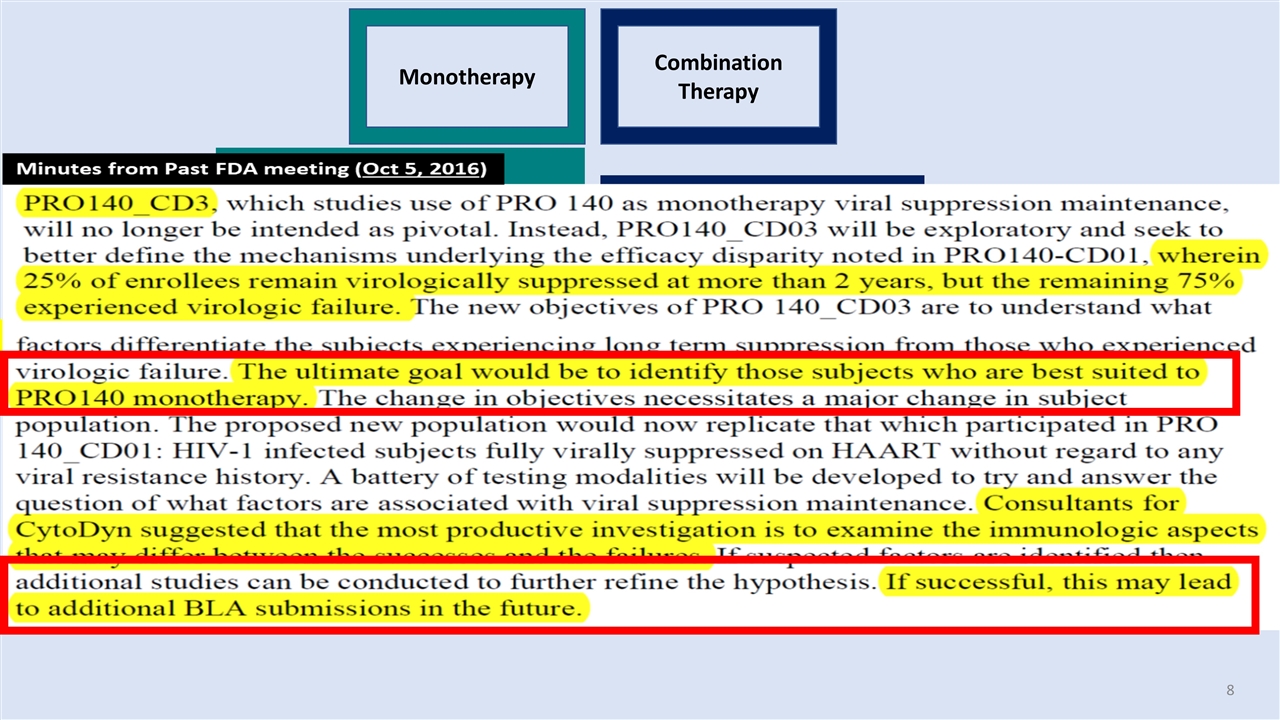
Monotherapy Combination Therapy Feb-2014 Phase 2 Feb-2015 Phase 3 pivotal Oct-2015 Phase 3 pivotal Oct-2015 1st patient Feb-2018 P.E. hit – p=0.0032 New Problems – If Monotherapy approval requested then we must solve the riddle – We did
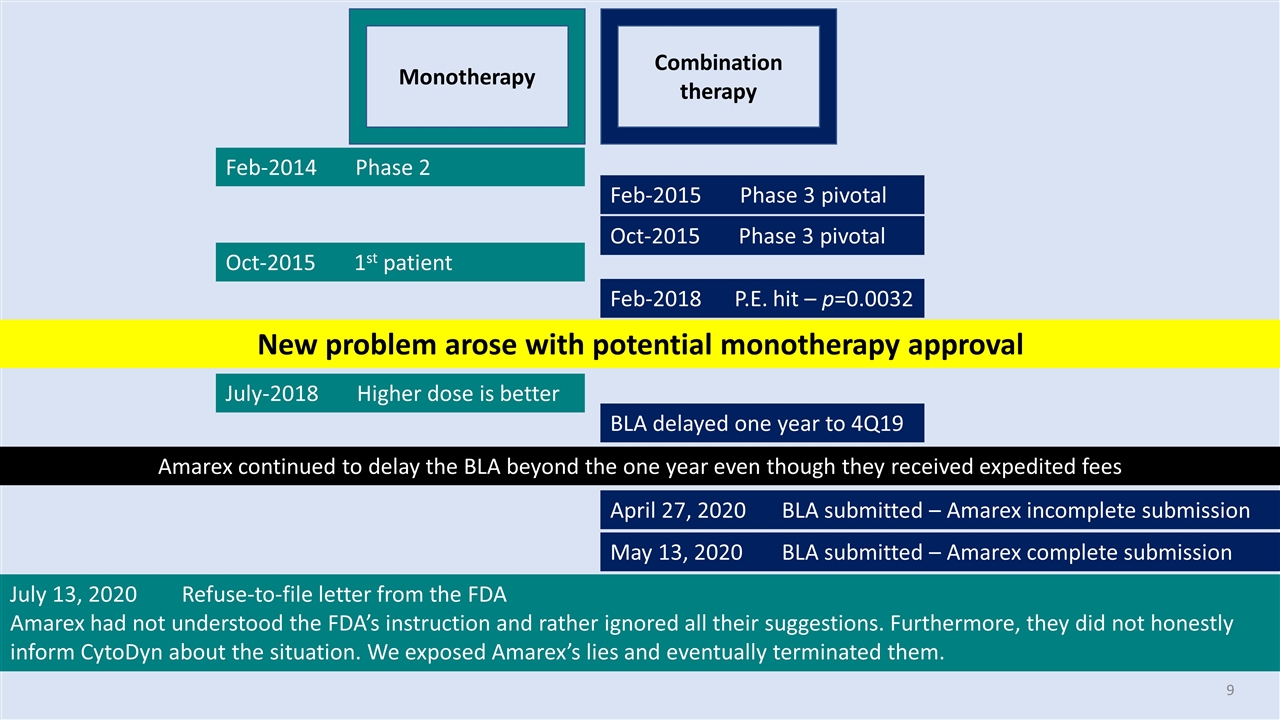
Monotherapy Combination therapy Feb-2014 Phase 2 Feb-2015 Phase 3 pivotal Oct-2015 Phase 3 pivotal Oct-2015 1st patient Feb-2018 P.E. hit – p=0.0032 Amarex continued to delay the BLA beyond the one year even though they received expedited fees July-2018 Higher dose is better BLA delayed one year to 4Q19 April 27, 2020 BLA submitted – Amarex incomplete submission May 13, 2020 BLA submitted – Amarex complete submission July 13, 2020 Refuse-to-file letter from the FDA Amarex had not understood the FDA’s instruction and rather ignored all their suggestions. Furthermore, they did not honestly inform CytoDyn about the situation. We exposed Amarex’s lies and eventually terminated them. New problem arose with potential monotherapy approval
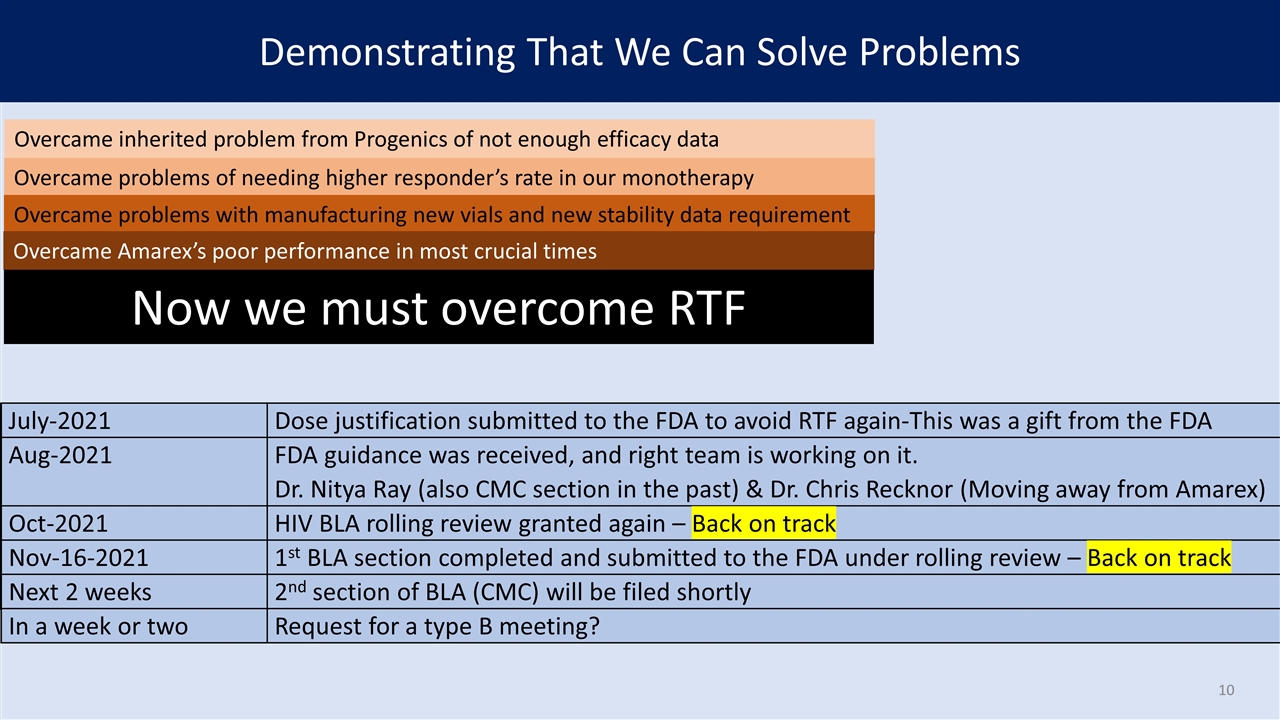
Demonstrating That We Can Solve Problems HIV Combination Therapy PrEP Monotherapy CURE July-2021 Dose justification submitted to the FDA to avoid RTF again-This was a gift from the FDA July-2021 Dose justification submitted to the FDA to avoid RTF again-This was a gift from the FDA Aug-2021 FDA guidance was received, and right team is working on it. Dr. Nitya Ray (also CMC section in the past) & Dr. Chris Recknor (moving away from Amarex) July-2021 Dose justification submitted to the FDA to avoid RTF again-This was a gift from the FDA Aug-2021 FDA guidance was received, and right team is working on it. Dr. Nitya Ray (also CMC section in the past) & Dr. Chris Recknor (moving away from Amarex) Oct-2021 HIV BLA rolling review granted again – Back on track July-2021 Dose justification submitted to the FDA to avoid RTF again-This was a gift from the FDA Aug-2021 FDA guidance was received, and right team is working on it. Dr. Nitya Ray (also CMC section in the past) & Dr. Chris Recknor (moving away from Amarex) Oct-2021 HIV BLA rolling review granted again – Back on track Nov-16-2021 1st BLA section completed and submitted to the FDA under rolling review – Back on track July-2021 Dose justification submitted to the FDA to avoid RTF again-This was a gift from the FDA Aug-2021 FDA guidance was received, and right team is working on it. Dr. Nitya Ray (also CMC section in the past) & Dr. Chris Recknor (moving away from Amarex) Oct-2021 HIV BLA rolling review granted again – Back on track Nov-16-2021 1st BLA section completed and submitted to the FDA under rolling review – Back on track Next 2 weeks 2nd section of BLA (CMC) will be filed shortly July-2021 Dose justification submitted to the FDA to avoid RTF again-This was a gift from the FDA Aug-2021 FDA guidance was received, and right team is working on it. Dr. Nitya Ray (also CMC section in the past) & Dr. Chris Recknor (Moving away from Amarex) Oct-2021 HIV BLA rolling review granted again – Back on track Nov-16-2021 1st BLA section completed and submitted to the FDA under rolling review – Back on track Next 2 weeks 2nd section of BLA (CMC) will be filed shortly In a week or two Request for a type B meeting? Overcame inherited problem from Progenics of not enough efficacy data Overcame problems of needing higher responder’s rate in our monotherapy Overcame problems with manufacturing new vials and new stability data requirement Overcame Amarex’s poor performance in most crucial times Now we must overcome RTF
Demonstrating That We Can Solve Problems HIV Combination Therapy PrEP Monotherapy CURE How about MANUFACTURING April 2, 2019 Samsung agreement - Side note: On 4/2/2019 CYDY=$0.52 How about MANUFACTURING April 2, 2019 Samsung agreement - Side note: On 4/2/2019 CYDY=$0.52 Signed a deal that had $20-30 million due very quickly – Our board members pushed back How about MANUFACTURING April 2, 2019 Samsung agreement - Side note: On 4/2/2019 CYDY=$0.52 Signed a deal that had $20-30 million due very quickly – Our board members pushed back The next 8 months CYDY decreased from $0.52 to $0.28 on 12/12/2019 CYDY=0.28 How about MANUFACTURING April 2, 2019 Samsung agreement - Side note: On 4/2/2019 CYDY=$0.52 Signed a deal that had $20-30 million due very quickly – Our board members pushed back The next 8 months CYDY decreased from $0.52 to $0.28 on 12/12/2019 CYDY=0.28 RECKLESS or GENIUS ? RECKLESS or GENIEUS?

Scientific Community Begin to Notice Leronlimab Success in HIV HIV Combination Therapy PrEP Monotherapy CURE Multiple presentations in ASM Microbe Multiple presentations at CROI (one of largest gatherings of top scientists in the world) Usually, we share our data with scientific community as soon as possible Many publications of leronlimab in the past 18 months

Abstract Presentations
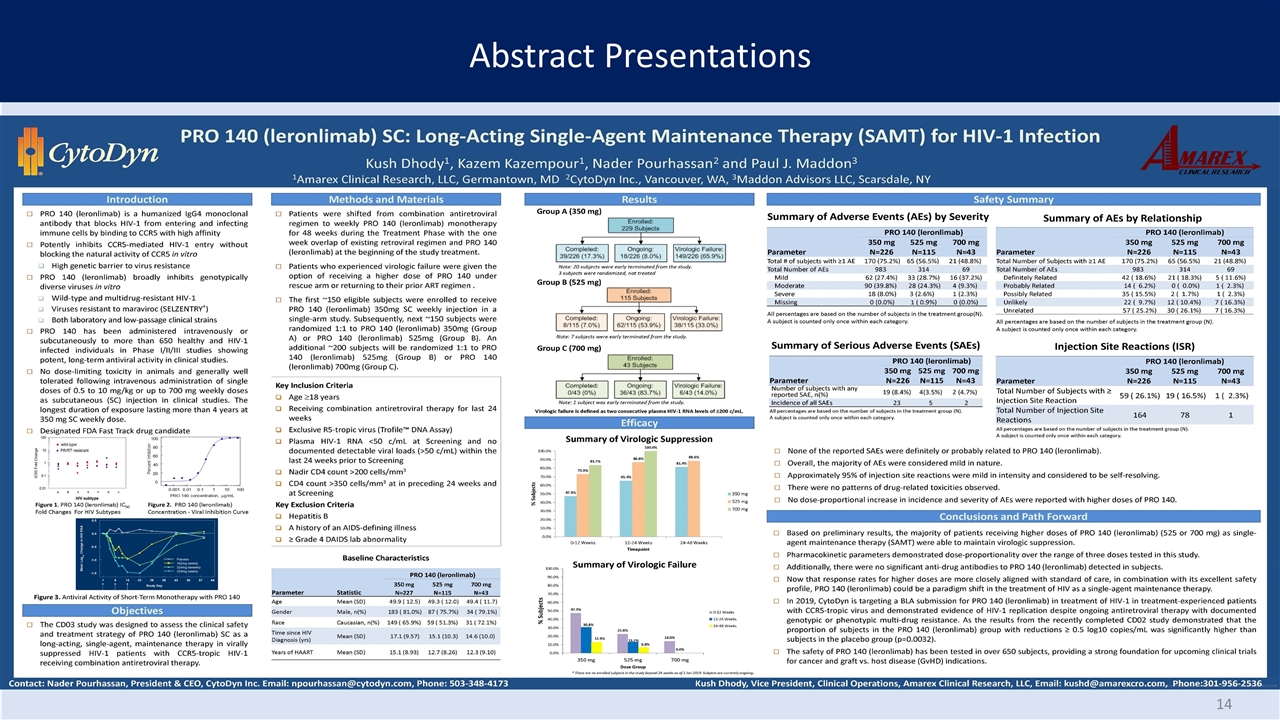
Abstract Presentations
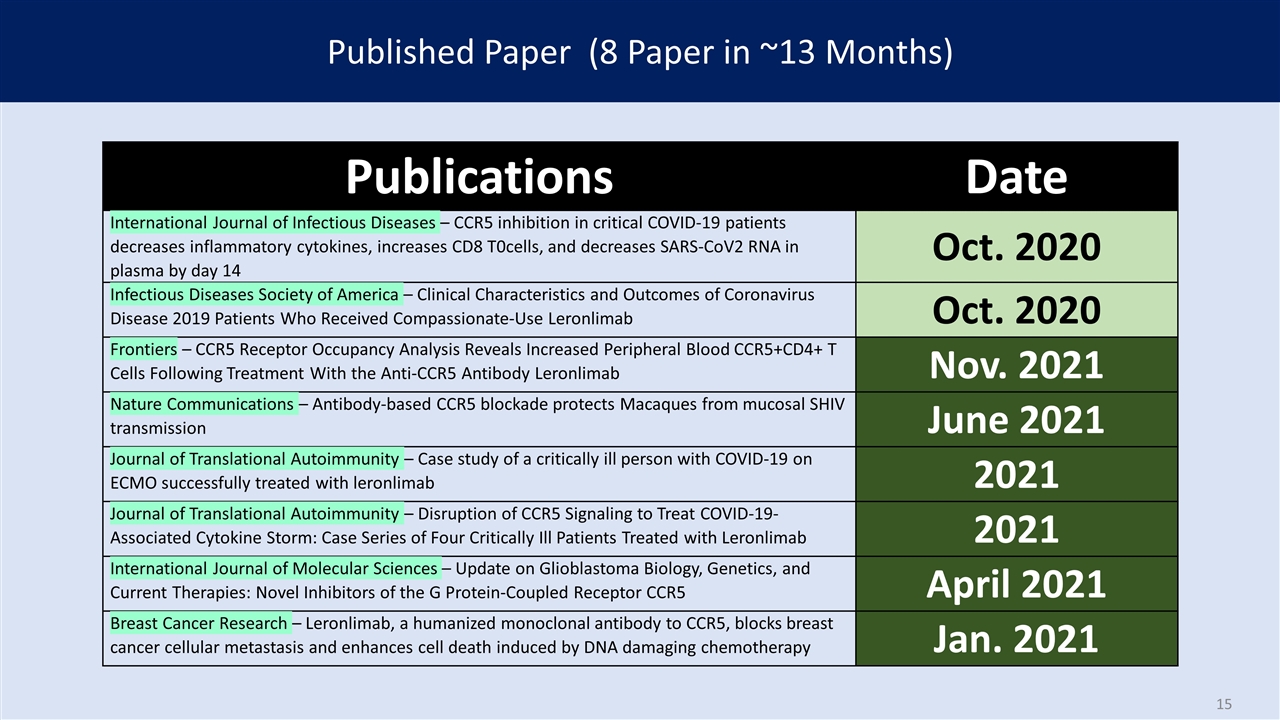
Published Paper (8 Paper in ~13 Months) Publications Date International Journal of Infectious Diseases – CCR5 inhibition in critical COVID-19 patients decreases inflammatory cytokines, increases CD8 T0cells, and decreases SARS-CoV2 RNA in plasma by day 14 Oct. 2020 Infectious Diseases Society of America – Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Patients Who Received Compassionate-Use Leronlimab Oct. 2020 Frontiers – CCR5 Receptor Occupancy Analysis Reveals Increased Peripheral Blood CCR5+CD4+ T Cells Following Treatment With the Anti-CCR5 Antibody Leronlimab Nov. 2021 Nature Communications – Antibody-based CCR5 blockade protects Macaques from mucosal SHIV transmission June 2021 Journal of Translational Autoimmunity – Case study of a critically ill person with COVID-19 on ECMO successfully treated with leronlimab 2021 Journal of Translational Autoimmunity – Disruption of CCR5 Signaling to Treat COVID-19-Associated Cytokine Storm: Case Series of Four Critically Ill Patients Treated with Leronlimab 2021 International Journal of Molecular Sciences – Update on Glioblastoma Biology, Genetics, and Current Therapies: Novel Inhibitors of the G Protein-Coupled Receptor CCR5 April 2021 Breast Cancer Research – Leronlimab, a humanized monoclonal antibody to CCR5, blocks breast cancer cellular metastasis and enhances cell death induced by DNA damaging chemotherapy Jan. 2021
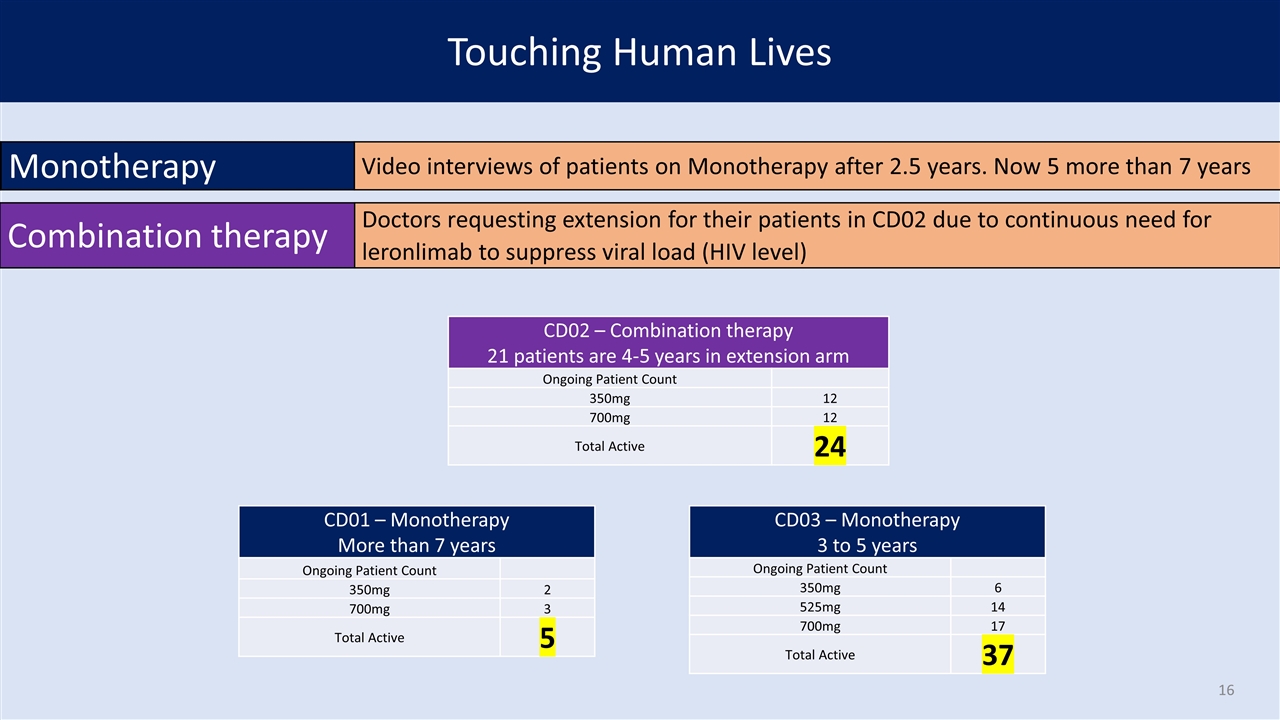
Touching Human Lives Monotherapy Video interviews of patients on Monotherapy after 2.5 years. Now 5 more than 7 years CD02 – Combination therapy 21 patients are 4-5 years in extension arm Ongoing Patient Count 350mg 12 700mg 12 Total Active 24 CD03 – Monotherapy 3 to 5 years Ongoing Patient Count 350mg 6 525mg 14 700mg 17 Total Active 37 CD01 – Monotherapy More than 7 years Ongoing Patient Count 350mg 2 700mg 3 Total Active 5 Combination therapy Doctors requesting extension for their patients in CD02 due to continuous need for leronlimab to suppress viral load (HIV level)
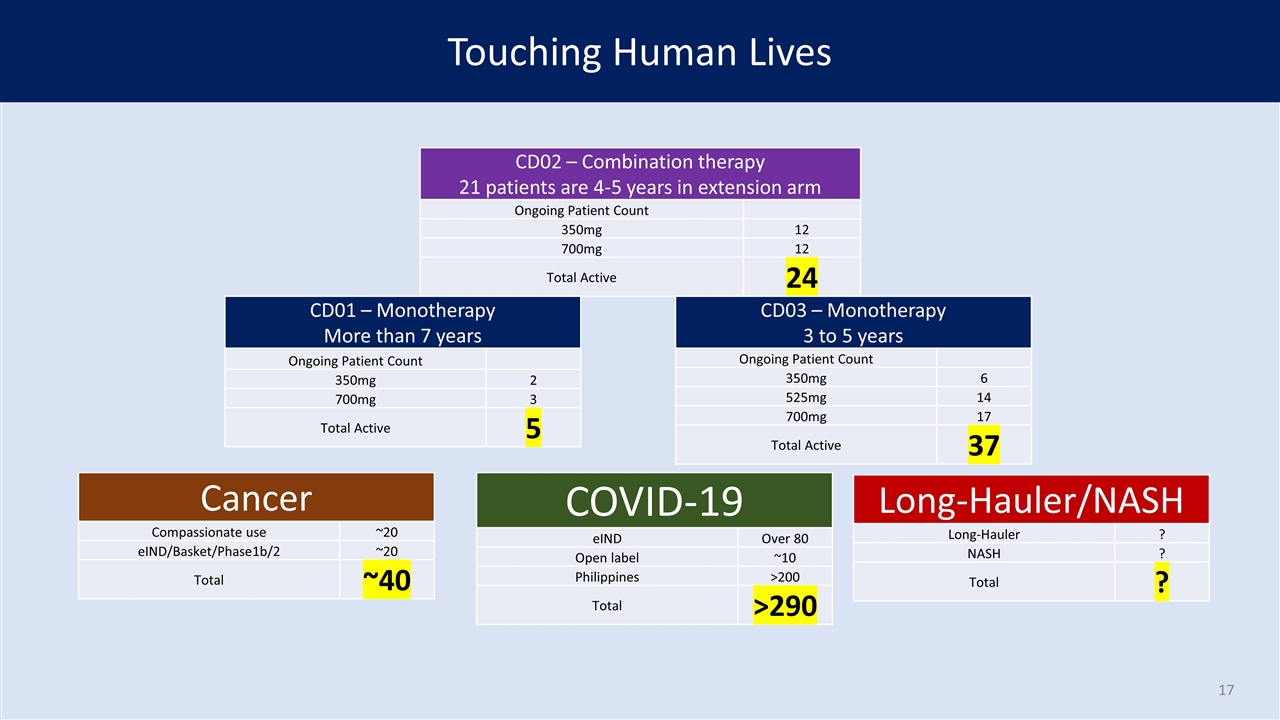
Touching Human Lives CD02 – Combination therapy 21 patients are 4-5 years in extension arm Ongoing Patient Count 350mg 12 700mg 12 Total Active 24 CD03 – Monotherapy 3 to 5 years Ongoing Patient Count 350mg 6 525mg 14 700mg 17 Total Active 37 CD01 – Monotherapy More than 7 years Ongoing Patient Count 350mg 2 700mg 3 Total Active 5 COVID-19 eIND Over 80 Open label ~10 Philippines >200 Total >290 Cancer Compassionate use ~20 eIND/Basket/Phase1b/2 ~20 Total ~40 Long-Hauler/NASH Long-Hauler ? NASH ? Total ?
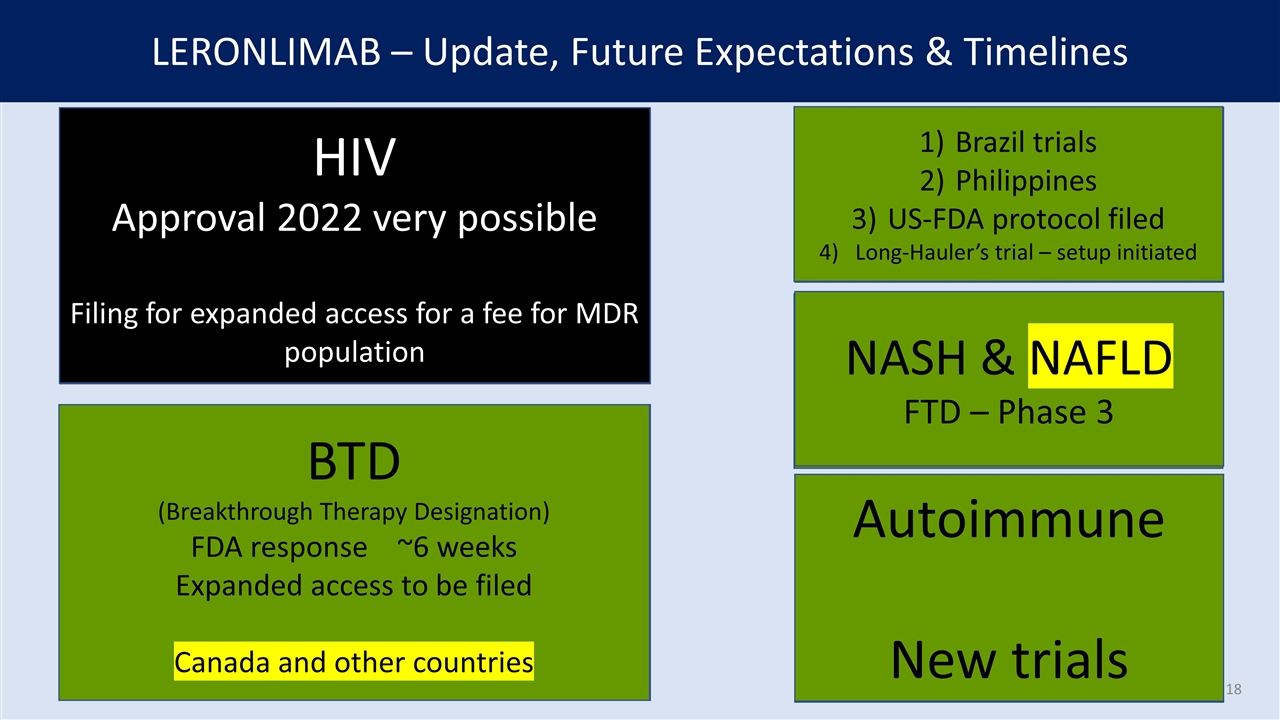
LERONLIMAB – Update, Future Expectations & Timelines NASH COVID Critically ill Long Hauler Severe HIV Combination Therapy PrEP Monotherapy CURE mTNBC COVID (3) Cancer (23) HIV (4) HIV Approval 2022 very possible Filing for expanded access for a fee for MDR population BTD (Breakthrough Therapy Designation) FDA response ~6 weeks Expanded access to be filed Canada and other countries Brazil trials Philippines US-FDA protocol filed Long-Hauler’s trial – setup initiated NASH (1) NASH & NAFLD FTD – Phase 3 Autoimmune (12) Autoimmune New trials Cancer BASKET TRIAL BreastUterine Colon CarcinomaTesticular OvarianSarcomas PancreasBladder ThyroidOther Endocrine AMLStomach Squamous head & neckMelanoma Brain-GlioblastomaThroat Blood/bone marrow CLL Liquid/blood tumors Hodgkin’s lymphomaLung EsophagusNon-Hodgkin’s lymp . AUTOIMMUNE DISEASES Crohn’s disease IBD (inflammatory bowel disease) Graves’ disease Hashimoto’s thyroiditis Autoimmune hepatitis Myasthenia gravis Sjogren’s syndrome Psoriasis Vasculitis MS Polymyositis

LERONLIMAB – From 1 Indication to 31 Indications in Just 7 years Potential Market Size From $50 Million to More Than $100 Billion Combination therapy for MDR ~ $30 million http://www.taimedbiologics.com/news/info/90#:~:text=A%20year%20ago%2C%20I%20was,based%20on%20Theratechnologies%27%20financial%20report. Combination therapy for 2 class resistance ~ $1 billion Monotherapy ~ $5 billion PrEP ~ $3 billion (https://www.managedhealthcareexecutive.com/view/how-has-the-covid-19-pandemic-affected-people-with-hiv ) From potential $30 million to $1 billion (2 class resistance) Then to $9 billion (MDR-Mono-PrEP) The global cancer therapy market was valued at approximately $158 billion in 2020, and it is expected to witness a revenue of USD $268 billion in 2026. Worldwide cancer data | World Cancer Research Fund International (wcrf.org) Potential $50 billion Potential market size for critical ill COVID-19 population maybe a few billion, however, long-hauler’s COVID-19 market size could be much larger than that. Potential $5 billion About $21 billions by 2023 https://www.reportsanddata.com/report-detail/non-alcoholic-steatohepatitis-nash-market The global NASH market was valued at USD 4.57 billion in 2020 and is projected to reach USD 180.09 billion by 2028, growing at a CAGR of 58.64%from 2020 to 2028 Potential $10 billion The Global Autoimmune Disease Therapeutics Market size is expected to reach $149.4 billion by 2025, rising at a market growth of 4.34% CAGR during the forecast period The Global Autoimmune Disease Therapeutics Market size is expected to reach $149.4 billion by 2025, rising at a market growth of 4.34% CAGR during the forecast period (prnewswire.com) Potential $50 billion 1 2 3 4 5 32 X Potential Partnership

Leronlimab - $3.5 Million Down Payment PRO 140 (leronlimab) was purchased for $3.5 million down payment Today CytoDyn has one molecule, leronlimab (potential 31 indications) And our Market cap is ~ $1 Billion (~300 times the down payment for leronlimab) And this is just the beginning 32 X
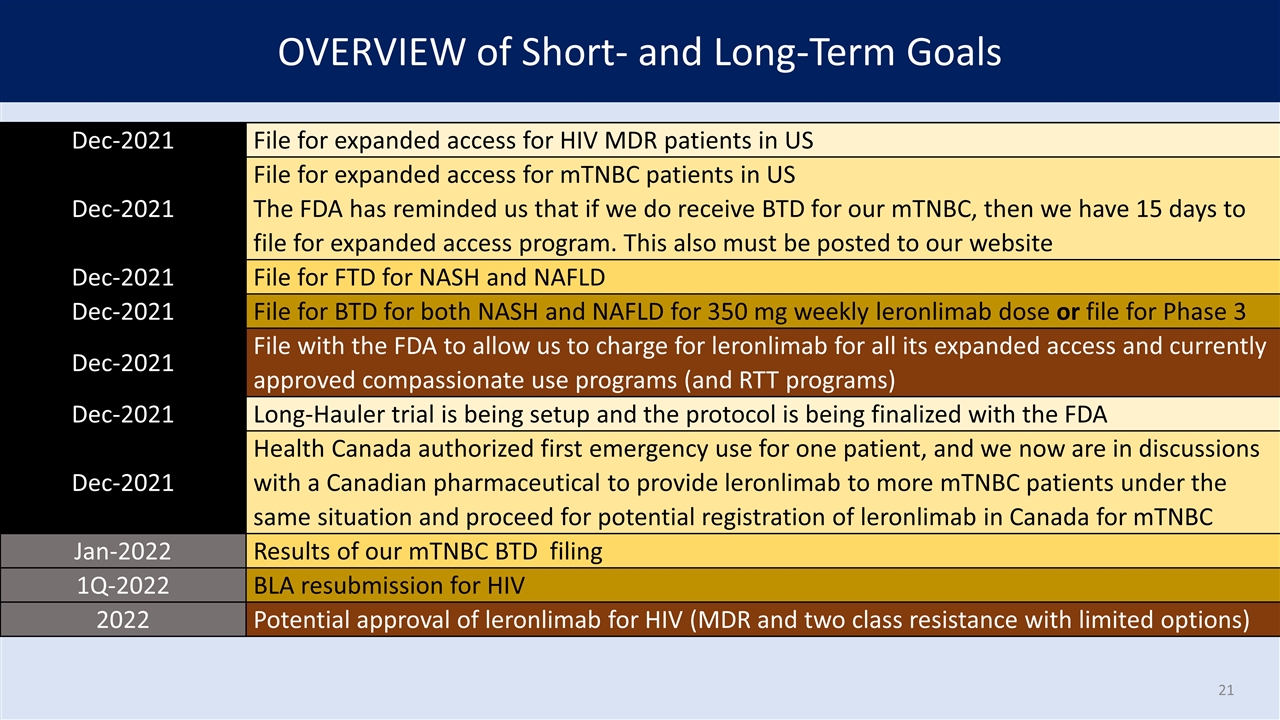
OVERVIEW of Short- and Long-Term Goals Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for FTD for NASH and NAFLD Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for FTD for NASH and NAFLD Dec-2021 File for BTD for both NASH and NAFLD for 350 mg weekly leronlimab dose Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for FTD for NASH and NAFLD Dec-2021 File for BTD for both NASH and NAFLD for 350 mg weekly leronlimab dose Dec-2021 File with the FDA to allow us to charge for leronlimab for all its expanded access and currently approved compassionate use programs (and RTT programs) Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for FTD for NASH and NAFLD Dec-2021 File for BTD for both NASH and NAFLD for 350 mg weekly leronlimab dose Dec-2021 File with the FDA to allow us to charge for leronlimab for all its expanded access and currently approved compassionate use programs (and RTT programs) Dec-2021 Long-Hauler trial is being setup and the protocol is being finalized with the FDA Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for FTD for NASH and NAFLD Dec-2021 File for BTD for both NASH and NAFLD for 350 mg weekly leronlimab dose Dec-2021 File with the FDA to allow us to charge for leronlimab for all its expanded access and currently approved compassionate use programs (and RTT programs) Dec-2021 Long-Hauler trial is being setup and the protocol is being finalized with the FDA Dec-2021 Health Canada authorized first emergency use for one patient, and we now are in discussions with a Canadian pharmaceutical to provide leronlimab to more mTNBC patients under the same situation and proceed for potential registration of leronlimab in Canada for mTNBC Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for FTD for NASH and NAFLD Dec-2021 File for BTD for both NASH and NAFLD for 350 mg weekly leronlimab dose Dec-2021 File with the FDA to allow us to charge for leronlimab for all its expanded access and currently approved compassionate use programs (and RTT programs) Dec-2021 Long-Hauler trial is being setup and the protocol is being finalized with the FDA Dec-2021 Health Canada authorized first emergency use for one patient, and we now are in discussions with a Canadian pharmaceutical to provide leronlimab to more mTNBC patients under the same situation and proceed for potential registration of leronlimab in Canada for mTNBC Jan-2022 Results of our mTNBC BTD filing Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for FTD for NASH and NAFLD Dec-2021 File for BTD for both NASH and NAFLD for 350 mg weekly leronlimab dose Dec-2021 File with the FDA to allow us to charge for leronlimab for all its expanded access and currently approved compassionate use programs (and RTT programs) Dec-2021 Long-Hauler trial is being setup and the protocol is being finalized with the FDA Dec-2021 Health Canada authorized first emergency use for one patient, and we now are in discussions with a Canadian pharmaceutical to provide leronlimab to more mTNBC patients under the same situation and proceed for potential registration of leronlimab in Canada for mTNBC Jan-2022 Results of our mTNBC BTD filing 1Q-2022 BLA resubmission for HIV Dec-2021 File for expanded access for HIV MDR patients in US Dec-2021 File for expanded access for mTNBC patients in US The FDA has reminded us that if we do receive BTD for our mTNBC, then we have 15 days to file for expanded access program. This also must be posted to our website Dec-2021 File for FTD for NASH and NAFLD Dec-2021 File for BTD for both NASH and NAFLD for 350 mg weekly leronlimab dose or file for Phase 3 Dec-2021 File with the FDA to allow us to charge for leronlimab for all its expanded access and currently approved compassionate use programs (and RTT programs) Dec-2021 Long-Hauler trial is being setup and the protocol is being finalized with the FDA Dec-2021 Health Canada authorized first emergency use for one patient, and we now are in discussions with a Canadian pharmaceutical to provide leronlimab to more mTNBC patients under the same situation and proceed for potential registration of leronlimab in Canada for mTNBC Jan-2022 Results of our mTNBC BTD filing 1Q-2022 BLA resubmission for HIV 2022 Potential approval of leronlimab for HIV (MDR and two class resistance with limited options)

Nader Z. Pourhassan, Ph.D. President, CEO and Director Executive Management Team Scott A. Kelly, M.D. Chief Medical Officer, Head of Business Development and Chairman of the Board Nitya G. Ray, Ph.D. Chief Operating and Technology Officer Antonio Migliarese, C.P.A. Chief Financial Officer Christopher P. Recknor, M.D. Senior Executive VP of Clinical Operations