

JULY 22, 2021 INVESTOR PRESENTATION Vyrologix (leronlimab – PRO 140) The pursuit of precision medicine Humanized Monoclonal Antibody CytoDyn Inc. Exhibit 99.2

Forward-Looking Statements & Information This presentation contains certain forward-looking statements that involve risks, uncertainties and assumptions that are difficult to predict. Words and expressions reflecting optimism, satisfaction or disappointment with current prospects, as well as words such as "believes," "hopes," "intends," "estimates," "expects," "projects," "plans," "anticipates" and variations thereof, or the use of future tense, identify forward-looking statements, but their absence does not mean that a statement is not forward-looking. Forward-looking statements specifically include statements about leronlimab, its ability to provide positive health outcomes, the possible results of clinical trials, studies or other programs or ability to continue those programs, the ability to obtain regulatory approval for commercial sales, and the market for actual commercial sales. The Company's forward-looking statements are not guarantees of performance, and actual results could vary materially from those contained in or expressed by such statements due to risks and uncertainties including: (i) the regulatory determination of leronlimab’s efficacy to treat COVID-19 by the U.S. Food and Drug Administration and various drug regulatory agencies in other countries, (ii) the Company's ability to raise additional capital to fund its operations, (iii) the Company's ability to meet its debt obligations, if any, (iv) the Company's ability to enter into partnership or licensing arrangements with third parties, (v) the Company's ability to identify patients to enroll in its clinical trials in a timely fashion, (vi) the Company's ability to achieve approval of a marketable product, (vii) the design, implementation and conduct of the Company's clinical trials, (viii) the results of the Company's clinical trials, including the possibility of unfavorable clinical trial results, (ix) the market for, and marketability of, any product that is approved, (x) the existence or development of vaccines, drugs, or other treatments that are viewed by medical professionals or patients as superior to the Company's products, (xi) regulatory initiatives, compliance with governmental regulations and the regulatory approval process, (xii) general economic and business conditions, (xiii) changes in foreign, political, and social conditions, and (xiv) various other matters, many of which are beyond the Company's control. The Company urges investors to consider specifically the various risk factors identified in its most recent Form 10-K, and any risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the Securities and Exchange Commission. Except as required by law, the Company does not undertake any responsibility to update any forward-looking statements to take into account events or circumstances that occur after the date of this presentation.
Robust Pipeline – Leronlimab 1) Cancer program mTNBC & Compassionate use Testimonies Follow up with FDA Basket trial 2) COVID-19 Long-Hauler Will send Phase 3 protocol to FDA Bio marker data 3) COVID-19 trials in Brazil (different definitions) Critical – 316 patients Severe - 612 patients 4) COVID-19 (Philippines – India) 5) BLA submission process Final timeline for completion (Oct-15-2021) 6) NASH trial CT1 and PDFF + Biomarker 7) Biomarker Lab Dr. Chris Recknor

Cancer Program – Leronlimab 1) Cancer program mTNBC & Compassionate use Testimonies Follow up with the FDA Basket trial

Preliminary Interim Task 1 Report: Pre-Analysis of mTNBC from Trial 756 (Compassionate Use) and Trial 706 (mTNBC) CytoDyn July 15th, 2021
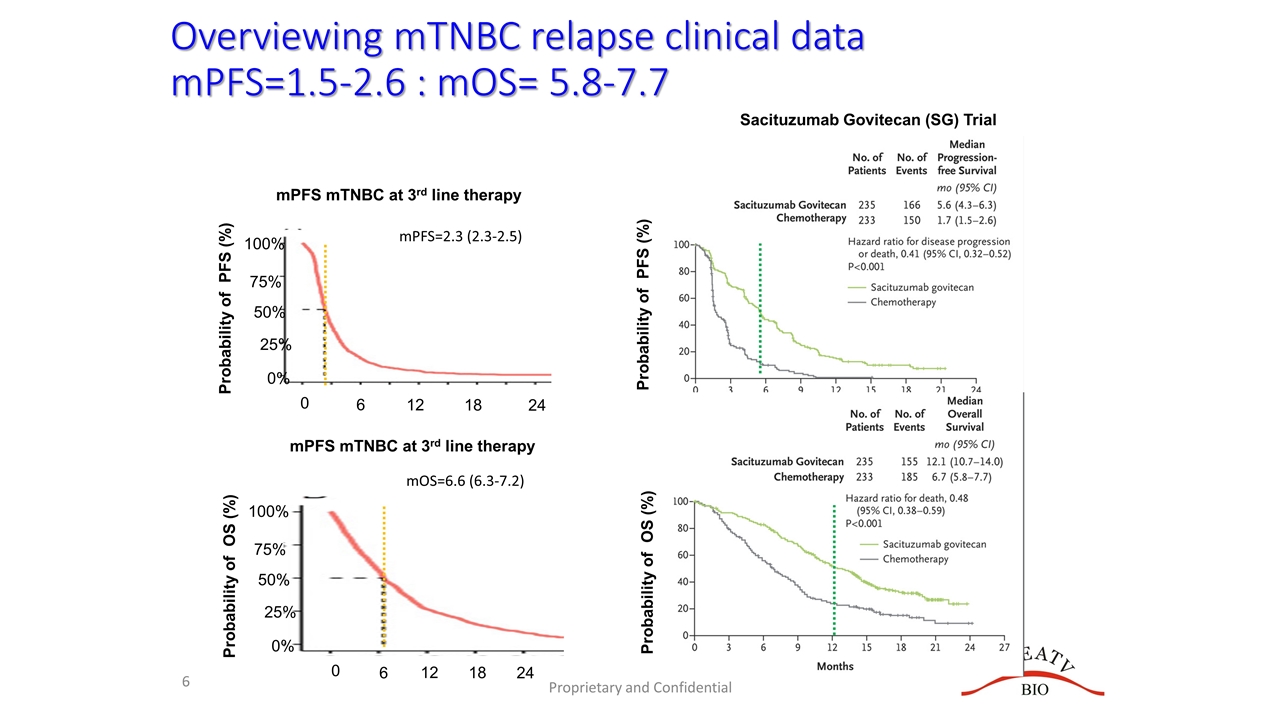
Overviewing mTNBC relapse clinical data mPFS=1.5-2.6 : mOS= 5.8-7.7 Proprietary and Confidential mPFS=2.3 (2.3-2.5) mOS=6.6 (6.3-7.2) Probability of OS (%) 0 75% 50% 25% 6 12 18 24 100% 0% Probability of PFS (%) 0 75% 50% 25% 6 12 18 24 100% 0% mPFS mTNBC at 3rd line therapy mPFS mTNBC at 3rd line therapy Sacituzumab Govitecan (SG) Trial Probability of OS (%) Probability of PFS (%)
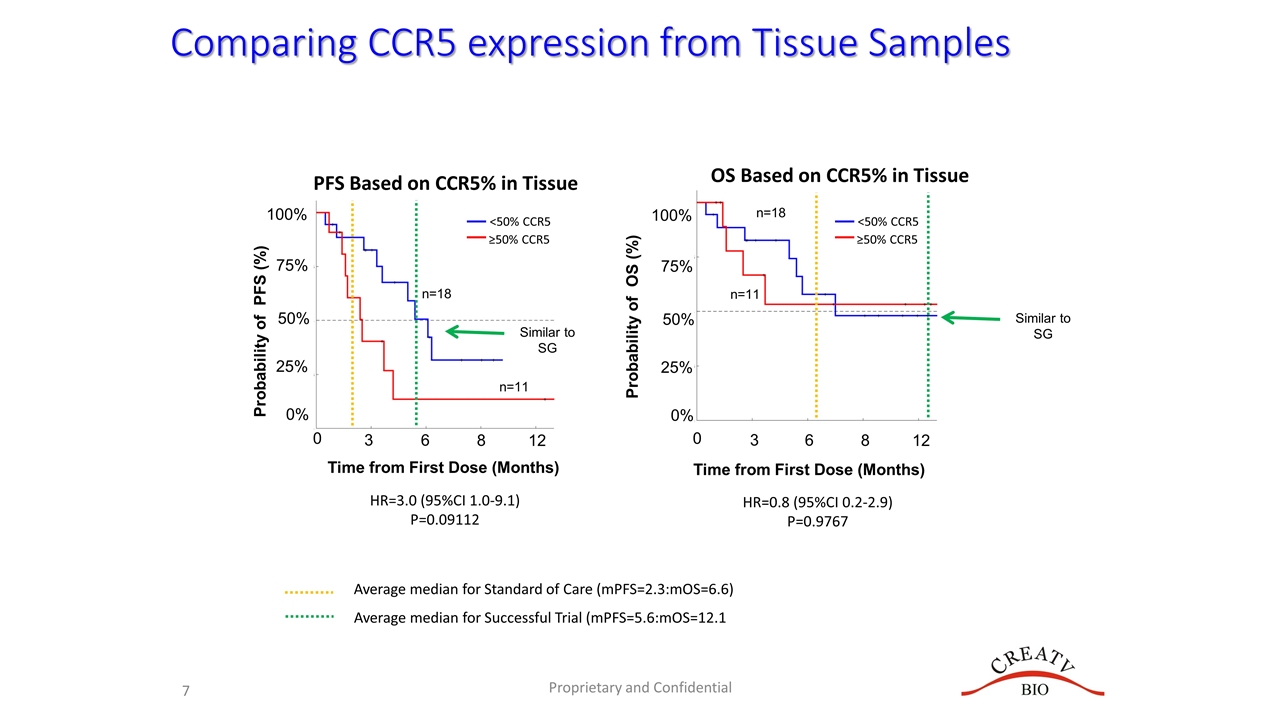
Comparing CCR5 expression from Tissue Samples Probability of PFS (%) 0 75% 50% 25% 0% 3 6 8 12 PFS Based on CCR5% in Tissue 100% Proprietary and Confidential OS Based on CCR5% in Tissue Time from First Dose (Months) 0 3 6 8 12 HR=3.0 (95%CI 1.0-9.1) P=0.09112 HR=0.8 (95%CI 0.2-2.9) P=0.9767 Probability of OS (%) 75% 50% 25% 0% 100% n=18 n=11 <50% CCR5 ≥50% CCR5 n=18 n=11 <50% CCR5 ≥50% CCR5 Time from First Dose (Months) Average median for Standard of Care (mPFS=2.3:mOS=6.6) Average median for Successful Trial (mPFS=5.6:mOS=12.1 Similar to SG Similar to SG
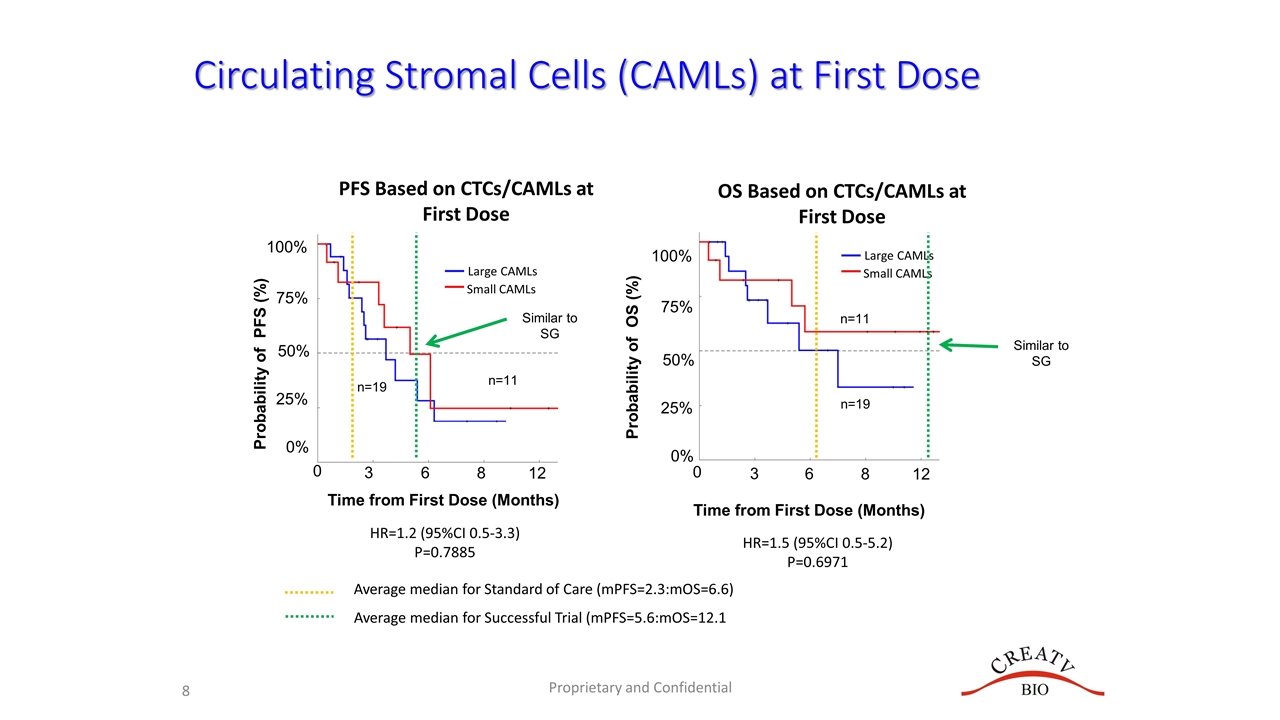
Circulating Stromal Cells (CAMLs) at First Dose Probability of PFS (%) 0 75% 50% 25% 0% 3 6 8 12 PFS Based on CTCs/CAMLs at First Dose 100% Proprietary and Confidential Time from First Dose (Months) 0 3 6 8 12 HR=1.2 (95%CI 0.5-3.3) P=0.7885 HR=1.5 (95%CI 0.5-5.2) P=0.6971 Probability of OS (%) 75% 50% 25% 0% 100% n=11 n=19 Large CAMLs Small CAMLs n=11 n=19 Time from First Dose (Months) OS Based on CTCs/CAMLs at First Dose Large CAMLs Small CAMLs Average median for Standard of Care (mPFS=2.3:mOS=6.6) Average median for Successful Trial (mPFS=5.6:mOS=12.1 Similar to SG Similar to SG
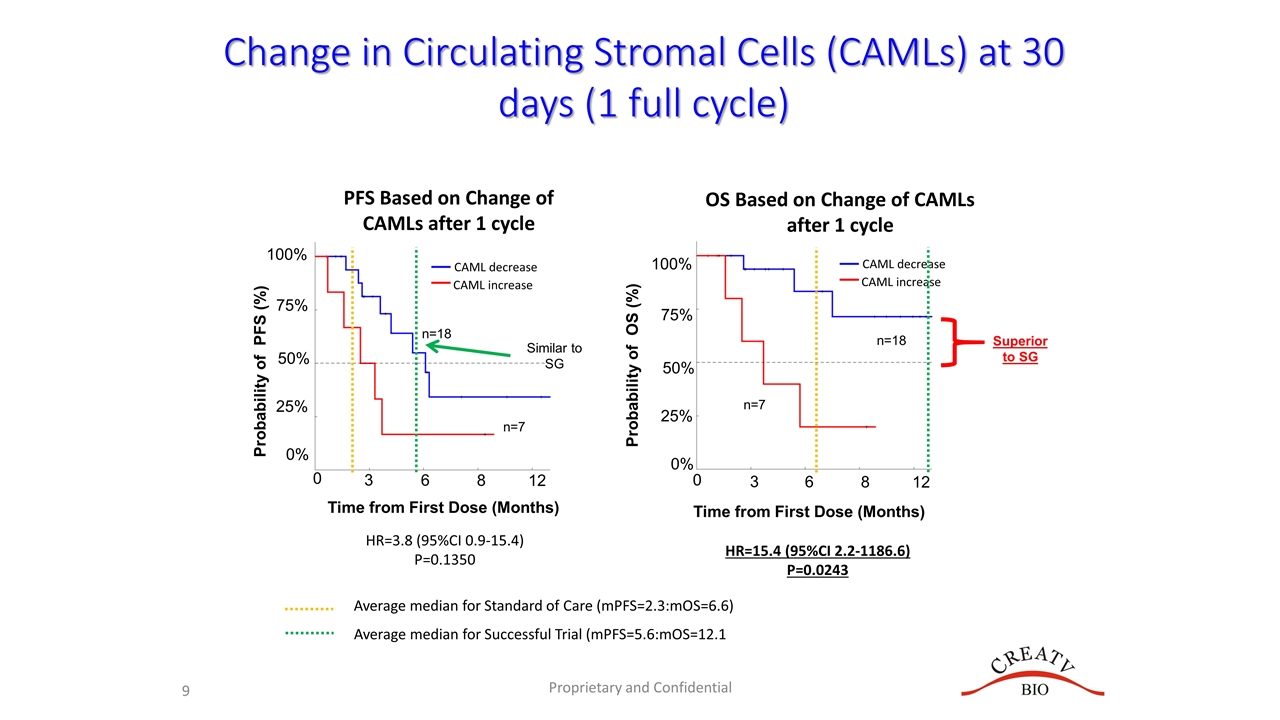
Probability of PFS (%) 0 75% 50% 25% 0% 3 6 8 12 100% Proprietary and Confidential Time from First Dose (Months) 0 3 6 8 12 HR=3.8 (95%CI 0.9-15.4) P=0.1350 HR=15.4 (95%CI 2.2-1186.6) P=0.0243 Probability of OS (%) 75% 50% 25% 0% 100% n=18 n=7 n=18 n=7 Time from First Dose (Months) OS Based on Change of CAMLs after 1 cycle CAML decrease CAML increase PFS Based on Change of CAMLs after 1 cycle Change in Circulating Stromal Cells (CAMLs) at 30 days (1 full cycle) CAML decrease CAML increase Average median for Standard of Care (mPFS=2.3:mOS=6.6) Average median for Successful Trial (mPFS=5.6:mOS=12.1 Similar to SG Superior to SG
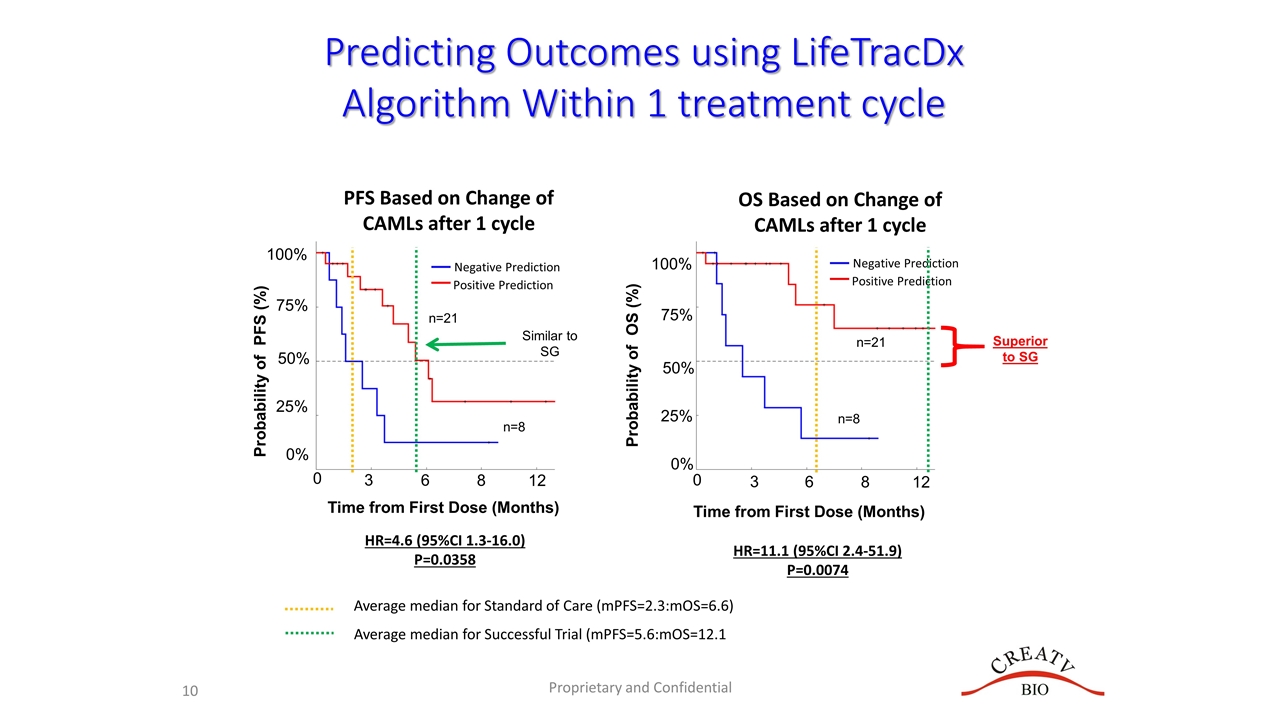
Probability of PFS (%) 0 75% 50% 25% 0% 3 6 8 12 100% Proprietary and Confidential Time from First Dose (Months) 0 3 6 8 12 HR=4.6 (95%CI 1.3-16.0) P=0.0358 HR=11.1 (95%CI 2.4-51.9) P=0.0074 Probability of OS (%) 75% 50% 25% 0% 100% n=21 n=8 n=21 n=8 Time from First Dose (Months) OS Based on Change of CAMLs after 1 cycle PFS Based on Change of CAMLs after 1 cycle Predicting Outcomes using LifeTracDx Algorithm Within 1 treatment cycle Negative Prediction Positive Prediction Negative Prediction Positive Prediction Average median for Standard of Care (mPFS=2.3:mOS=6.6) Average median for Successful Trial (mPFS=5.6:mOS=12.1 Similar to SG Superior to SG
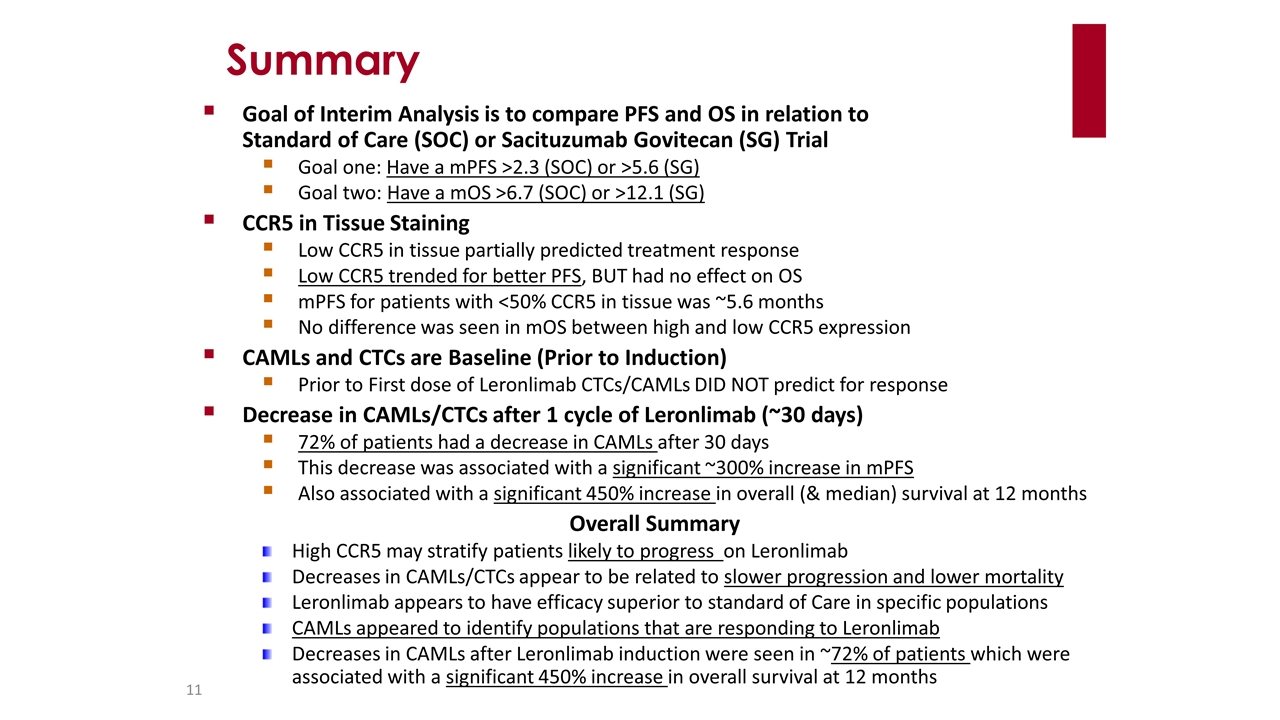
Summary Goal of Interim Analysis is to compare PFS and OS in relation to Standard of Care (SOC) or Sacituzumab Govitecan (SG) Trial Goal one: Have a mPFS >2.3 (SOC) or >5.6 (SG) Goal two: Have a mOS >6.7 (SOC) or >12.1 (SG) CCR5 in Tissue Staining Low CCR5 in tissue partially predicted treatment response Low CCR5 trended for better PFS, BUT had no effect on OS mPFS for patients with <50% CCR5 in tissue was ~5.6 months No difference was seen in mOS between high and low CCR5 expression CAMLs and CTCs are Baseline (Prior to Induction) Prior to First dose of Leronlimab CTCs/CAMLs DID NOT predict for response Decrease in CAMLs/CTCs after 1 cycle of Leronlimab (~30 days) 72% of patients had a decrease in CAMLs after 30 days This decrease was associated with a significant ~300% increase in mPFS Also associated with a significant 450% increase in overall (& median) survival at 12 months Overall Summary High CCR5 may stratify patients likely to progress on Leronlimab Decreases in CAMLs/CTCs appear to be related to slower progression and lower mortality Leronlimab appears to have efficacy superior to standard of Care in specific populations CAMLs appeared to identify populations that are responding to Leronlimab Decreases in CAMLs after Leronlimab induction were seen in ~72% of patients which were associated with a significant 450% increase in overall survival at 12 months
COVID-19 Long-Hauler - Leronlimab Responses to COVID-19 (1) Appropriate inflammation response balanced by appropriate healing. A normal release of cytokines causes blood vessel walls to become leakier to promote healing of damaged tissue via inflammation. (2) Inappropriately high inflammation A cytokine storm may occur when too many pathogens enter the body at once, or if the body secretes the wrong type of cytokine early in the immune response, in which case the excessive cytokines can't accurately direct the immune system to clear out the pathogen. Because nearly every organ has cytokine receptors, almost every part of the body is susceptible to the negative effects of a cytokine storm. Excess cytokines cause blood vessels to become overly porous and result in low blood pressure. That, in turn, depletes organs of oxygen and could eventually cause death (3) Inappropriate low inflammation with inappropriately high healing. Dysregulated immune function and to imbalance of cytokines Possible reactivation of Virus EBV, HSV, etc./ disruption of normal commensal bacteria 24 symptoms examined in the CD15 trial along with cytokine and cellular biomarkers
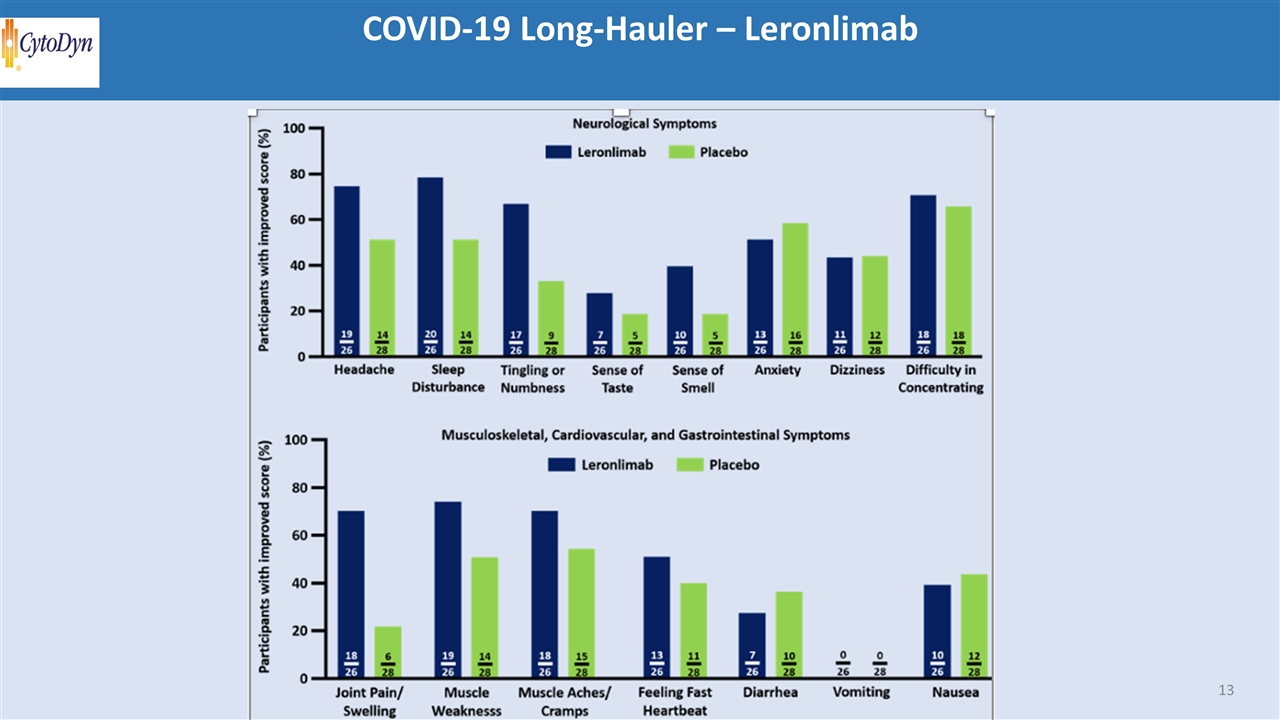
COVID-19 Long-Hauler – Leronlimab

COVID-19 Long-Hauler – Leronlimab

COVID-19 Long-Hauler – Leronlimab
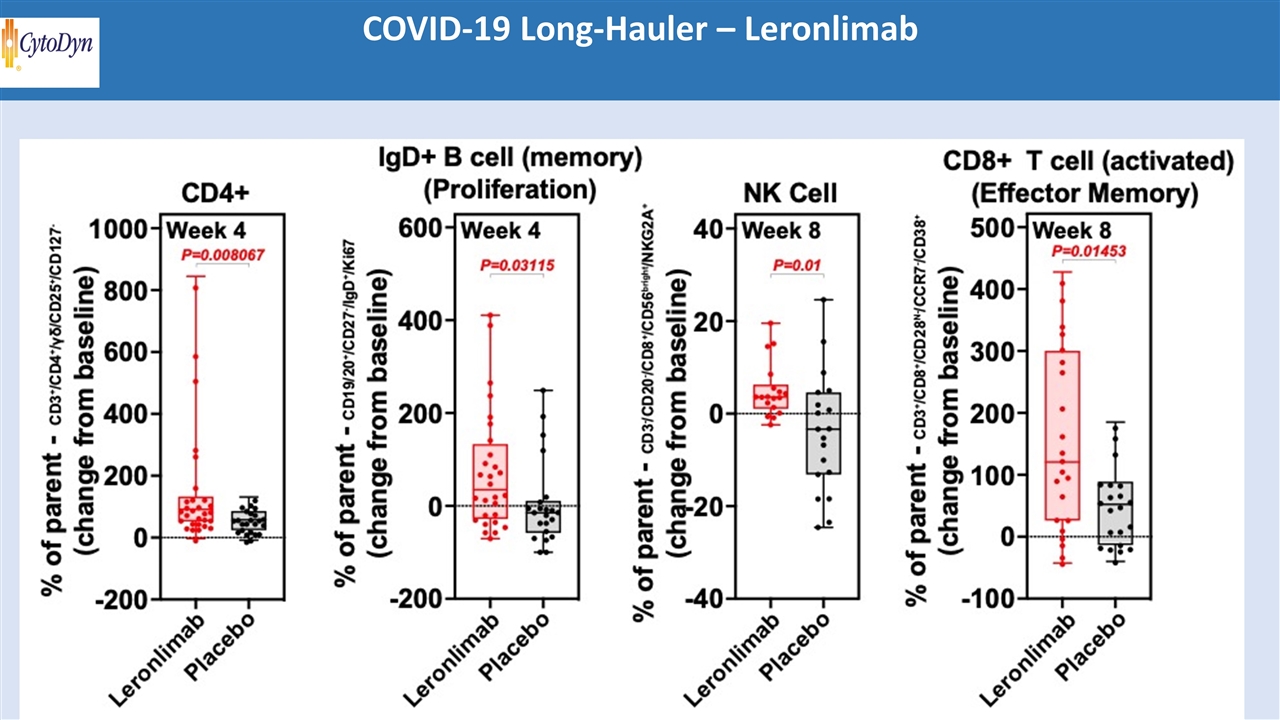
COVID-19 Long-Hauler – Leronlimab

COVID-19 Long-Hauler – Leronlimab
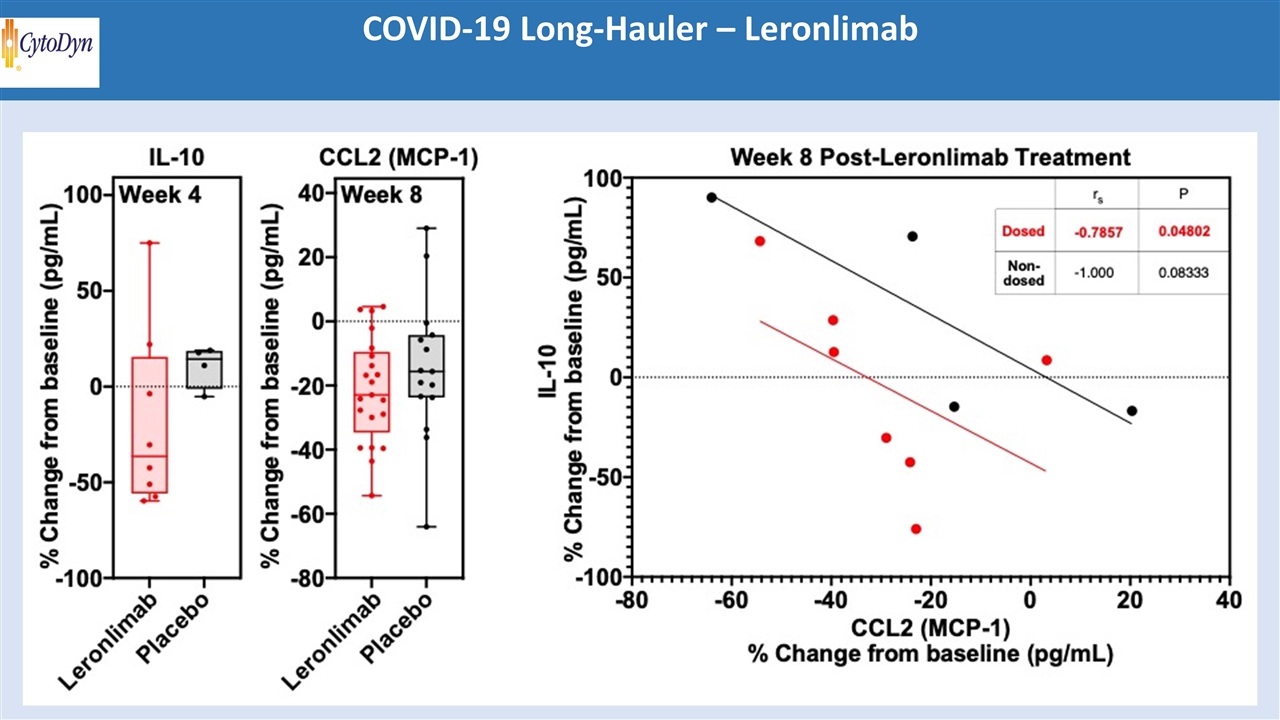
COVID-19 Long-Hauler – Leronlimab
COVID-19 Long-Hauler - Leronlimab Results: Symptom improvement noted on daily diary Baseline dysregulated Immune function in control and treated groups Down regulated T cells Immune system shifted Th2 M2 monocyte polarization predominant up to 66% Biomarkers correlating with symptom improvement Restoration of immune function Immune system shifted Th1 M2 monocyte polarization reduced
COVID-19 Trials – Leronlimab 3) COVID-19 trials in Brazil (different definitions) Critical – 316 patients Severe - 612 patients 4) COVID-19 (Philippines – India)

COVID-19 Trials – Leronlimab 5) BLA submission process Final timeline for completion (Oct-15-2021)
NASH Trial– Leronlimab 6) NASH trial CT1 and PDFF + Biomarker

Diagnostic Lab for CytoDyn by Dr. Recknor – Leronlimab 7) Biomarker Diagnostic test Lab Dr. Chris Recknor a) Receptor Occupancy Test