
JUNE 21, 2021 INVESTOR PRESENTATION Vyrologix (leronlimab – PRO 140) The pursuit of precision medicine Humanized Monoclonal Antibody CytoDyn Inc. Exhibit 99.2

Forward-Looking Statements & Information This presentation contains certain forward-looking statements that involve risks, uncertainties and assumptions that are difficult to predict. Words and expressions reflecting optimism, satisfaction or disappointment with current prospects, as well as words such as "believes," "hopes," "intends," "estimates," "expects," "projects," "plans," "anticipates" and variations thereof, or the use of future tense, identify forward-looking statements, but their absence does not mean that a statement is not forward-looking. Forward-looking statements specifically include statements about leronlimab, its ability to provide positive health outcomes, the possible results of clinical trials, studies or other programs or ability to continue those programs, the ability to obtain regulatory approval for commercial sales, and the market for actual commercial sales. The Company's forward-looking statements are not guarantees of performance, and actual results could vary materially from those contained in or expressed by such statements due to risks and uncertainties including: (i) the regulatory determination of leronlimab’s efficacy to treat COVID-19 by the U.S. Food and Drug Administration and various drug regulatory agencies in other countries, (ii) the Company's ability to raise additional capital to fund its operations, (iii) the Company's ability to meet its debt obligations, if any, (iv) the Company's ability to enter into partnership or licensing arrangements with third parties, (v) the Company's ability to identify patients to enroll in its clinical trials in a timely fashion, (vi) the Company's ability to achieve approval of a marketable product, (vii) the design, implementation and conduct of the Company's clinical trials, (viii) the results of the Company's clinical trials, including the possibility of unfavorable clinical trial results, (ix) the market for, and marketability of, any product that is approved, (x) the existence or development of vaccines, drugs, or other treatments that are viewed by medical professionals or patients as superior to the Company's products, (xi) regulatory initiatives, compliance with governmental regulations and the regulatory approval process, (xii) general economic and business conditions, (xiii) changes in foreign, political, and social conditions, and (xiv) various other matters, many of which are beyond the Company's control. The Company urges investors to consider specifically the various risk factors identified in its most recent Form 10-K, and any risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the Securities and Exchange Commission. Except as required by law, the Company does not undertake any responsibility to update any forward-looking statements to take into account events or circumstances that occur after the date of this presentation.
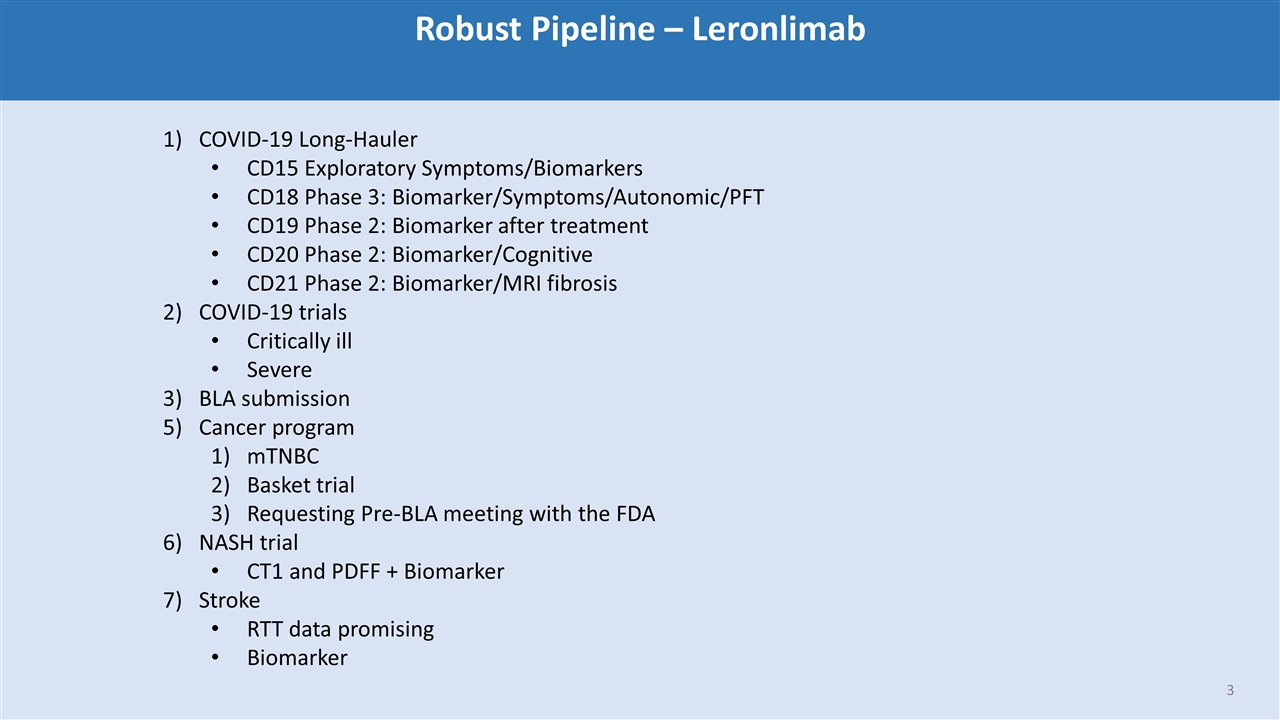
Robust Pipeline – Leronlimab COVID-19 Long-Hauler CD15 Exploratory Symptoms/Biomarkers CD18 Phase 3: Biomarker/Symptoms/Autonomic/PFT CD19 Phase 2: Biomarker after treatment CD20 Phase 2: Biomarker/Cognitive CD21 Phase 2: Biomarker/MRI fibrosis COVID-19 trials Critically ill Severe BLA submission Cancer program mTNBC Basket trial Requesting Pre-BLA meeting with the FDA NASH trial CT1 and PDFF + Biomarker Stroke RTT data promising Biomarker
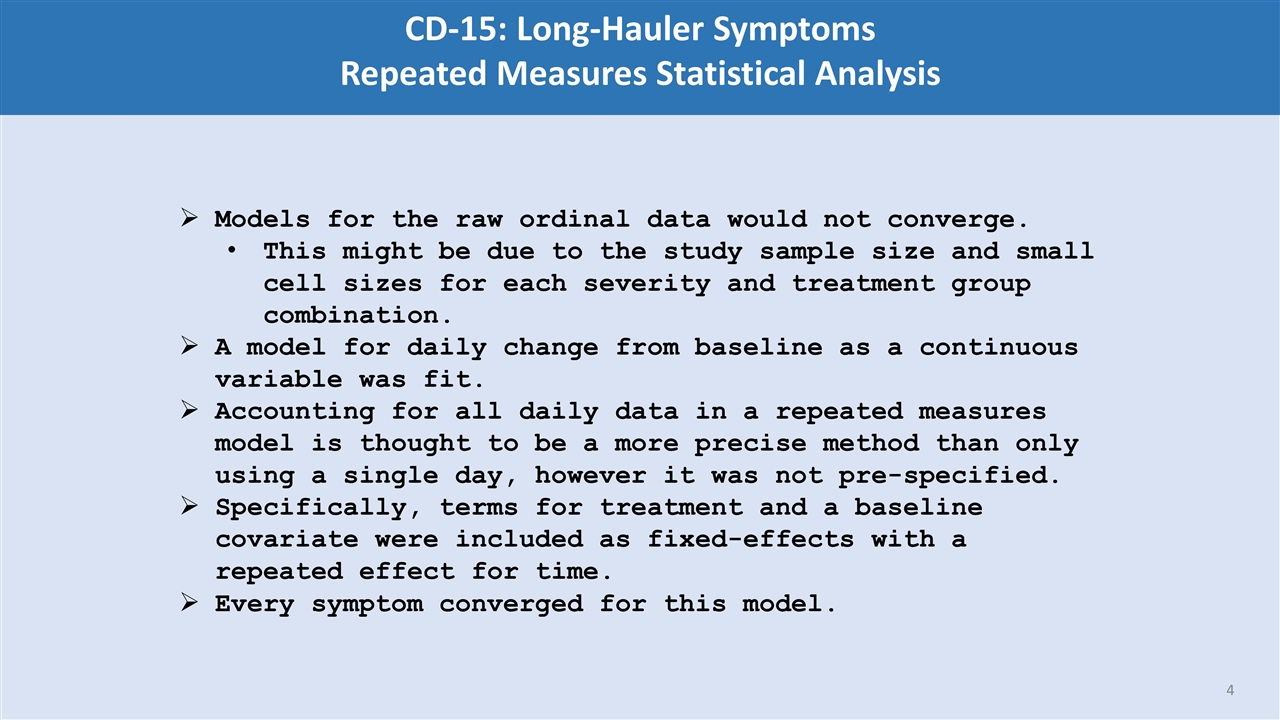
Models for the raw ordinal data would not converge. This might be due to the study sample size and small cell sizes for each severity and treatment group combination. A model for daily change from baseline as a continuous variable was fit. Accounting for all daily data in a repeated measures model is thought to be a more precise method than only using a single day, however it was not pre-specified. Specifically, terms for treatment and a baseline covariate were included as fixed-effects with a repeated effect for time. Every symptom converged for this model. CD-15: Long-Hauler Symptoms Repeated Measures Statistical Analysis
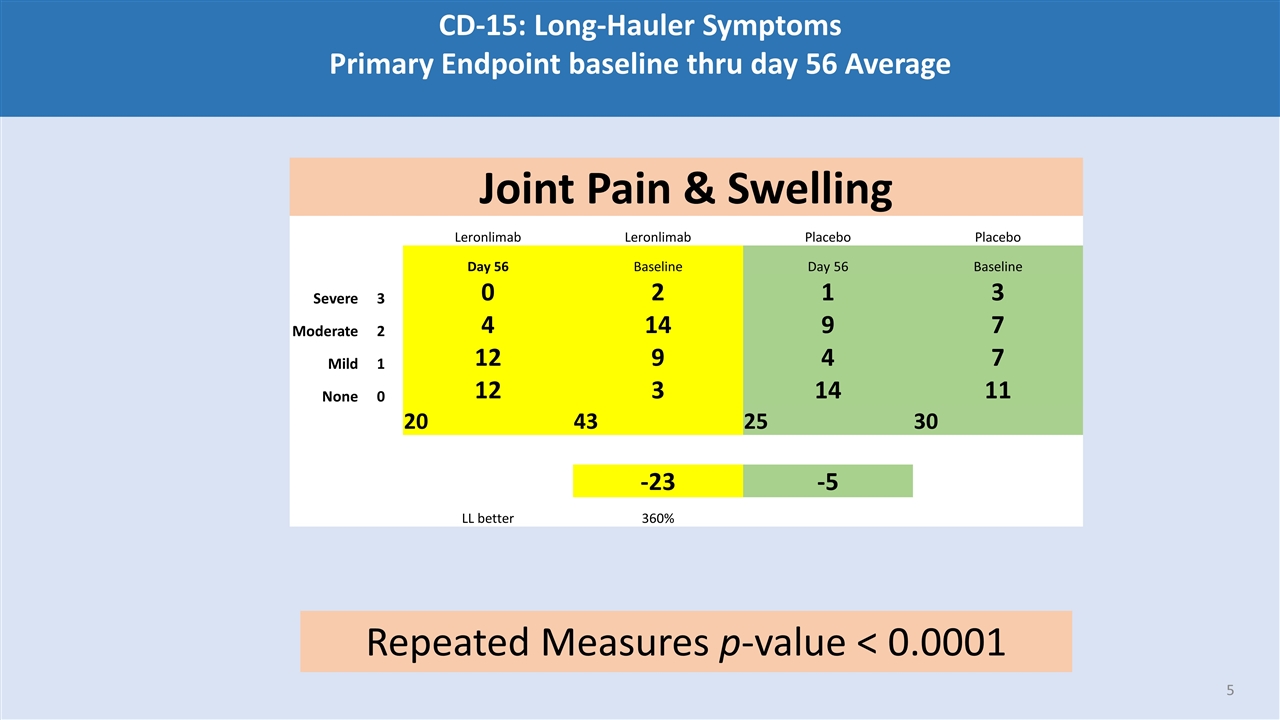
CD-15: Long-Hauler Symptoms Primary Endpoint baseline thru day 56 Average Joint Pain & Swelling Leronlimab Leronlimab Placebo Placebo Day 56 Baseline Day 56 Baseline Severe 3 0 2 1 3 Moderate 2 4 14 9 7 Mild 1 12 9 4 7 None 0 12 3 14 11 20 43 25 30 -23 -5 LL better 360% Repeated Measures p-value < 0.0001
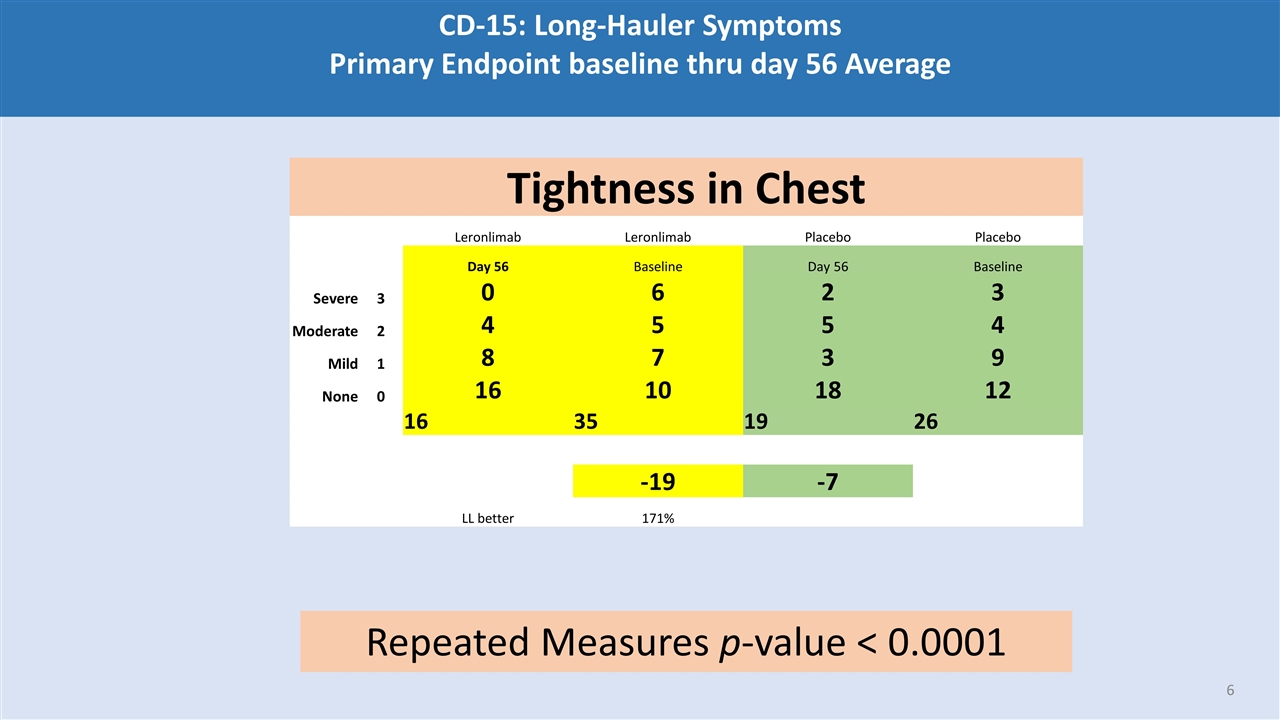
CD-15: Long-Hauler Symptoms Primary Endpoint baseline thru day 56 Average Tightness in Chest Leronlimab Leronlimab Placebo Placebo Day 56 Baseline Day 56 Baseline Severe 3 0 6 2 3 Moderate 2 4 5 5 4 Mild 1 8 7 3 9 None 0 16 10 18 12 16 35 19 26 -19 -7 LL better 171% Repeated Measures p-value < 0.0001
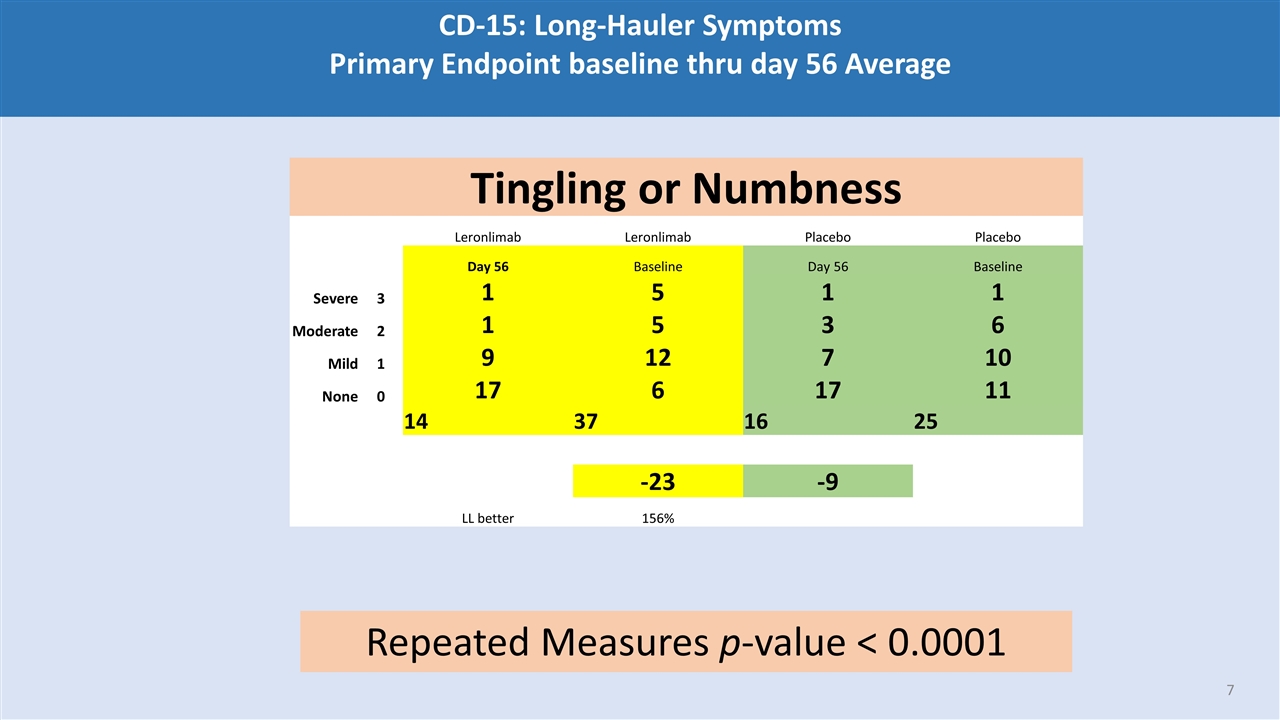
CD-15: Long-Hauler Symptoms Primary Endpoint baseline thru day 56 Average Tingling or Numbness Leronlimab Leronlimab Placebo Placebo Day 56 Baseline Day 56 Baseline Severe 3 1 5 1 1 Moderate 2 1 5 3 6 Mild 1 9 12 7 10 None 0 17 6 17 11 14 37 16 25 -23 -9 LL better 156% Repeated Measures p-value < 0.0001
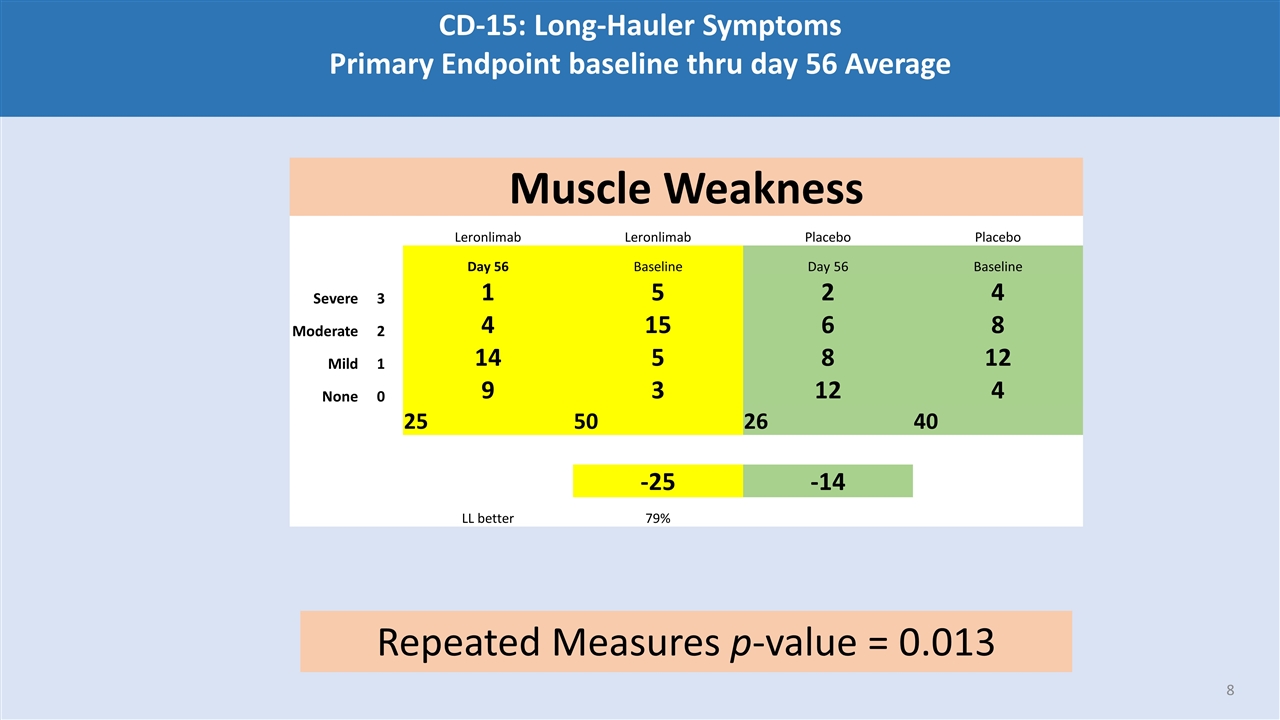
CD-15: Long-Hauler Symptoms Primary Endpoint baseline thru day 56 Average Muscle Weakness Leronlimab Leronlimab Placebo Placebo Day 56 Baseline Day 56 Baseline Severe 3 1 5 2 4 Moderate 2 4 15 6 8 Mild 1 14 5 8 12 None 0 9 3 12 4 25 50 26 40 -25 -14 LL better 79% Repeated Measures p-value = 0.013
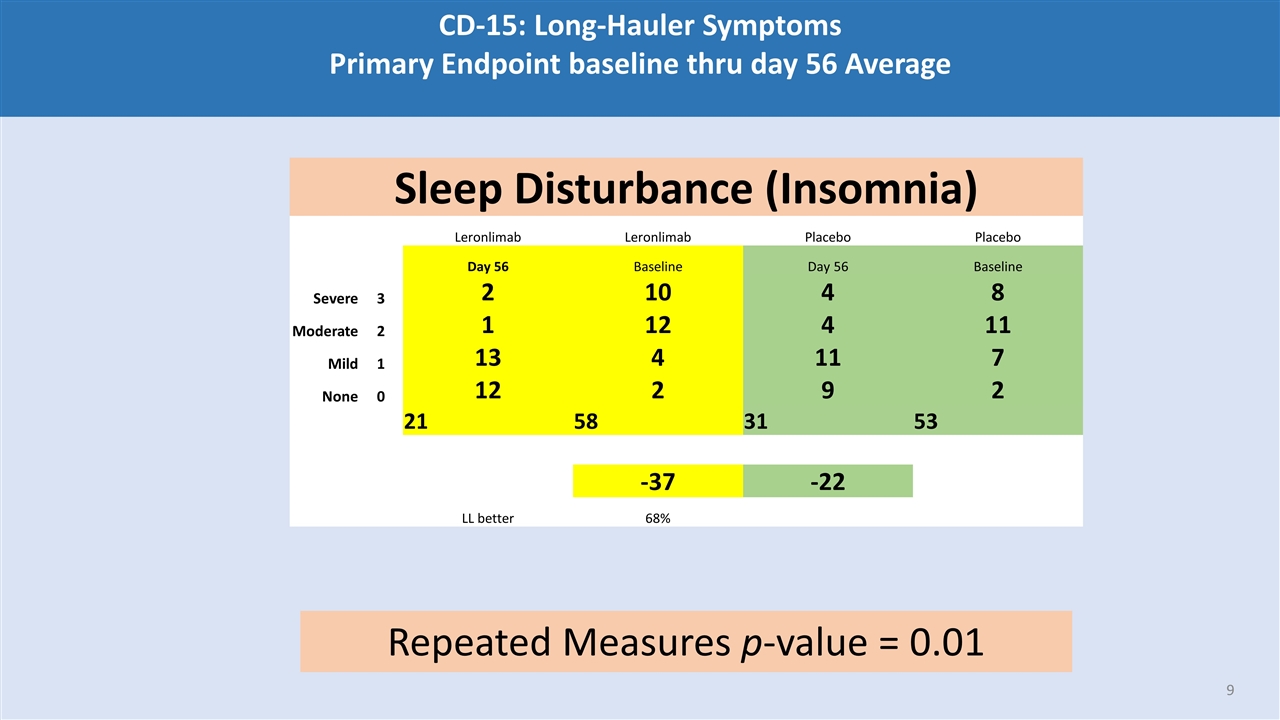
CD-15: Long-Hauler Symptoms Primary Endpoint baseline thru day 56 Average Sleep Disturbance (Insomnia) Leronlimab Leronlimab Placebo Placebo Day 56 Baseline Day 56 Baseline Severe 3 2 10 4 8 Moderate 2 1 12 4 11 Mild 1 13 4 11 7 None 0 12 2 9 2 21 58 31 53 -37 -22 LL better 68% Repeated Measures p-value = 0.01
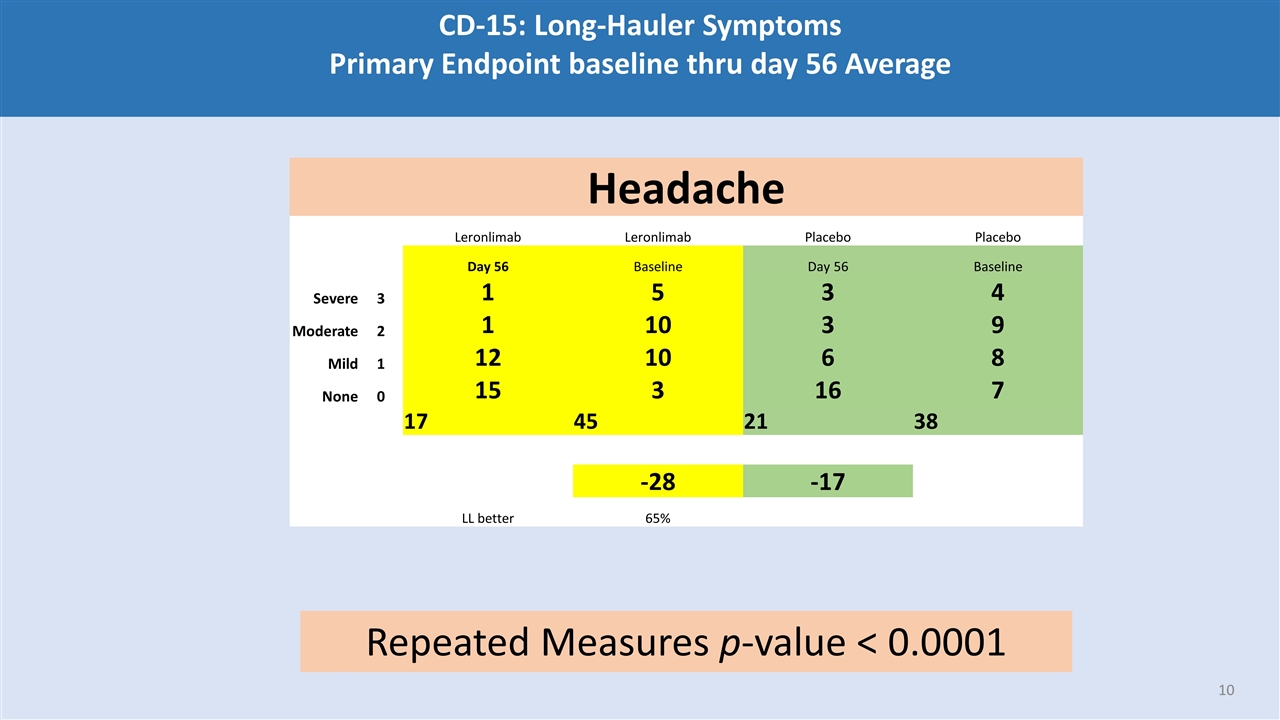
CD-15: Long-Hauler Symptoms Primary Endpoint baseline thru day 56 Average Headache Leronlimab Leronlimab Placebo Placebo Day 56 Baseline Day 56 Baseline Severe 3 1 5 3 4 Moderate 2 1 10 3 9 Mild 1 12 10 6 8 None 0 15 3 16 7 17 45 21 38 -28 -17 LL better 65% Repeated Measures p-value < 0.0001
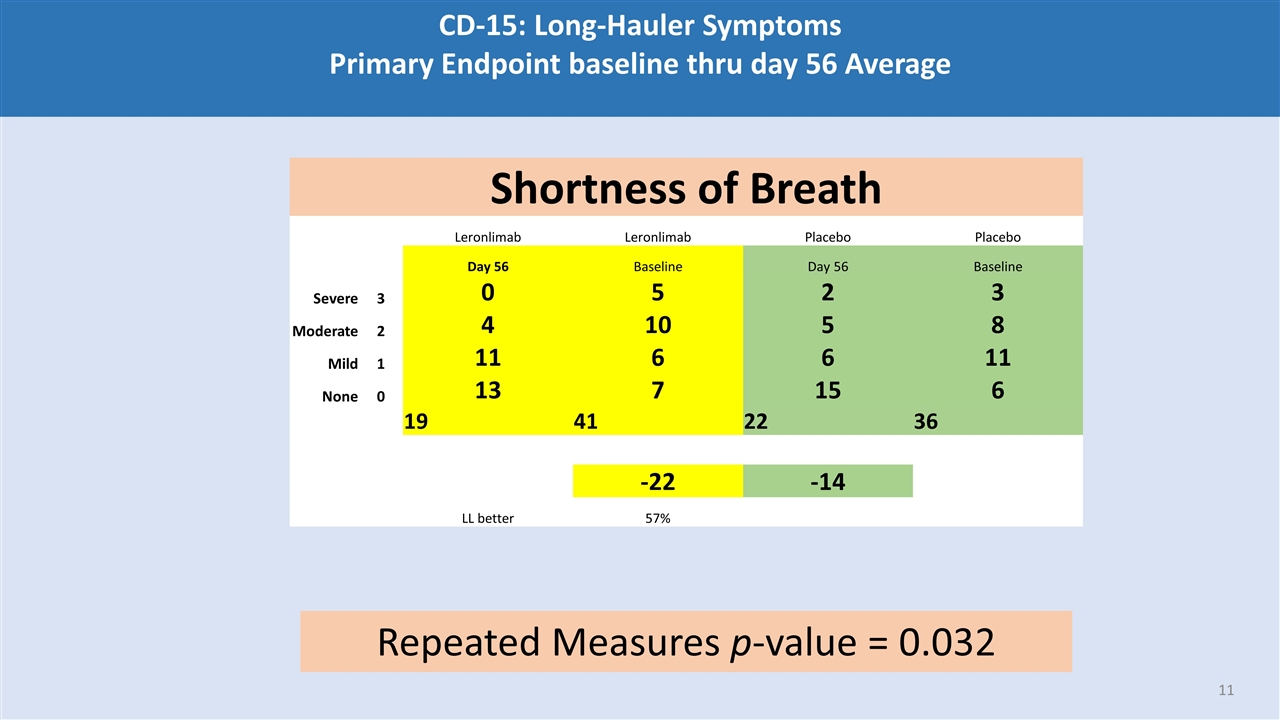
CD-15: Long-Hauler Symptoms Primary Endpoint baseline thru day 56 Average Shortness of Breath Leronlimab Leronlimab Placebo Placebo Day 56 Baseline Day 56 Baseline Severe 3 0 5 2 3 Moderate 2 4 10 5 8 Mild 1 11 6 6 11 None 0 13 7 15 6 19 41 22 36 -22 -14 LL better 57% Repeated Measures p-value = 0.032
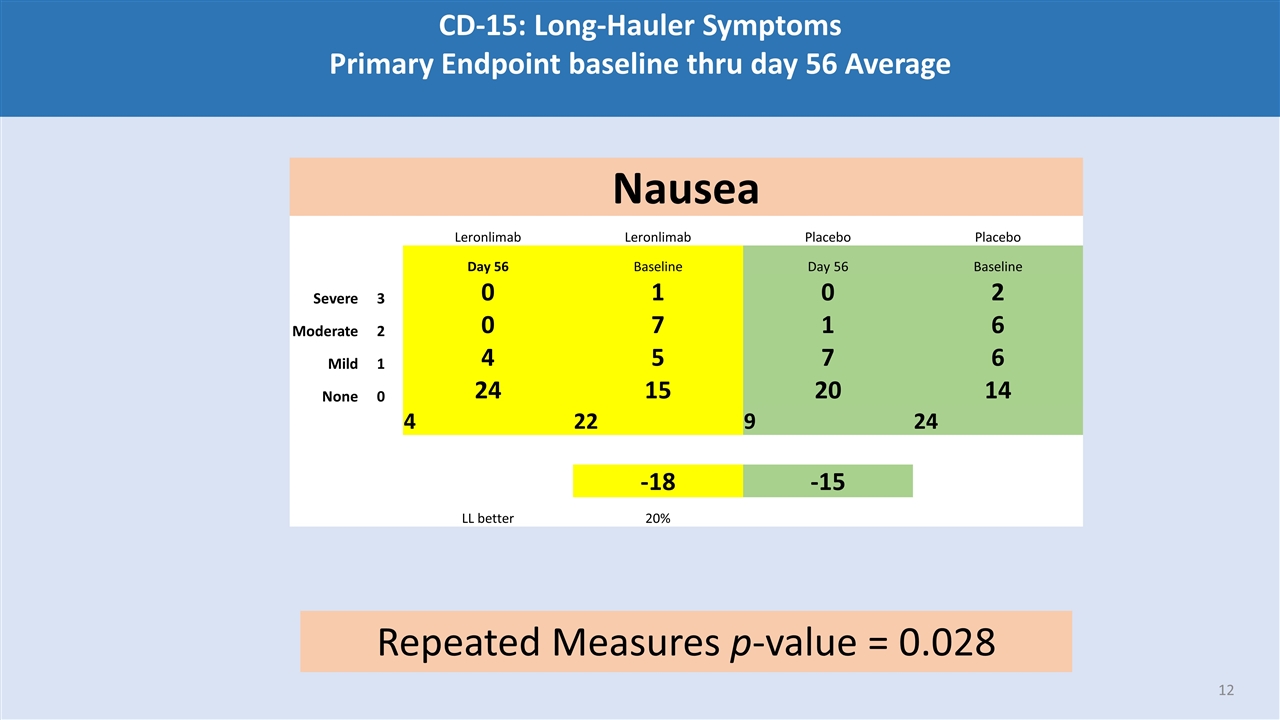
CD-15: Long-Hauler Symptoms Primary Endpoint baseline thru day 56 Average Nausea Leronlimab Leronlimab Placebo Placebo Day 56 Baseline Day 56 Baseline Severe 3 0 1 0 2 Moderate 2 0 7 1 6 Mild 1 4 5 7 6 None 0 24 15 20 14 4 22 9 24 -18 -15 LL better 20% Repeated Measures p-value = 0.028
Robust Pipeline – Leronlimab COVID-19 Long-Hauler COVID-19 Critically ill COVID-19 Severe Brazil India Philippines Other

Robust Pipeline – Leronlimab Biomarker and Mechanism of Action Laboratory work finding MOA of leronlimab for each indication Dosage of leronlimab
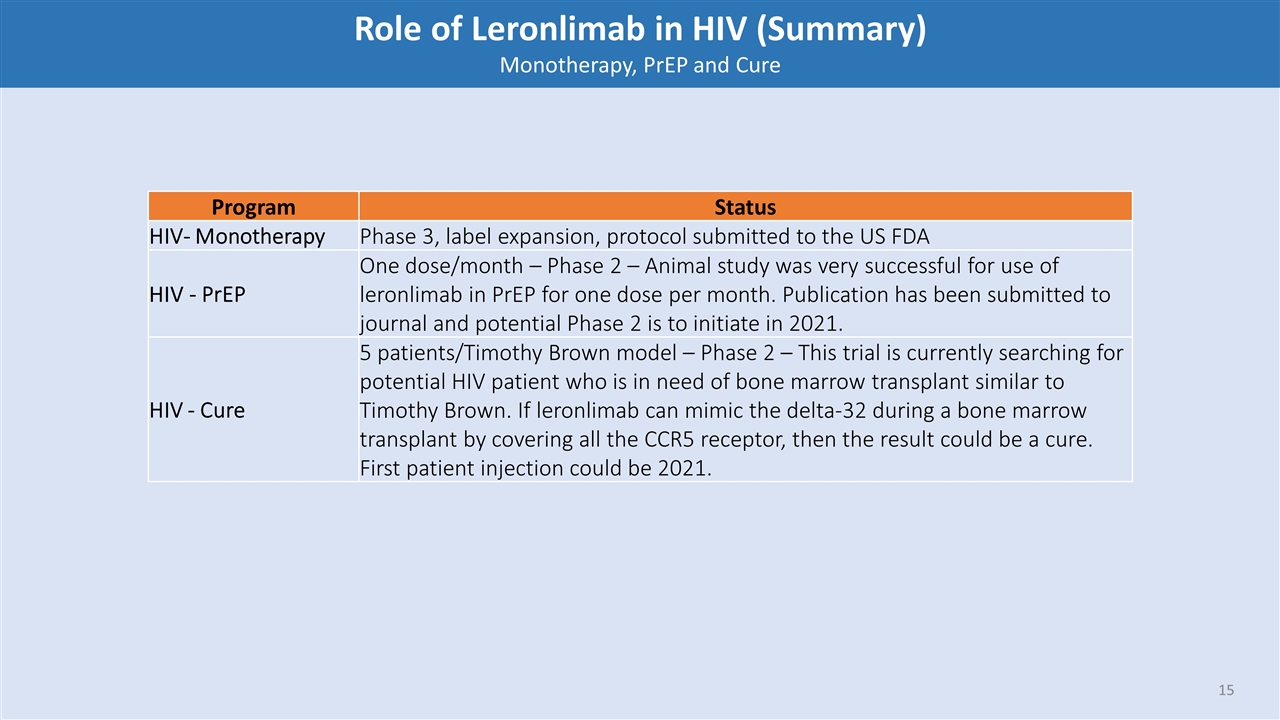
Program Status HIV- Monotherapy Phase 3, label expansion, protocol submitted to the US FDA HIV - PrEP One dose/month – Phase 2 – Animal study was very successful for use of leronlimab in PrEP for one dose per month. Publication has been submitted to journal and potential Phase 2 is to initiate in 2021. HIV - Cure 5 patients/Timothy Brown model – Phase 2 – This trial is currently searching for potential HIV patient who is in need of bone marrow transplant similar to Timothy Brown. If leronlimab can mimic the delta-32 during a bone marrow transplant by covering all the CCR5 receptor, then the result could be a cure. First patient injection could be 2021. Role of Leronlimab in HIV (Summary) Monotherapy, PrEP and Cure
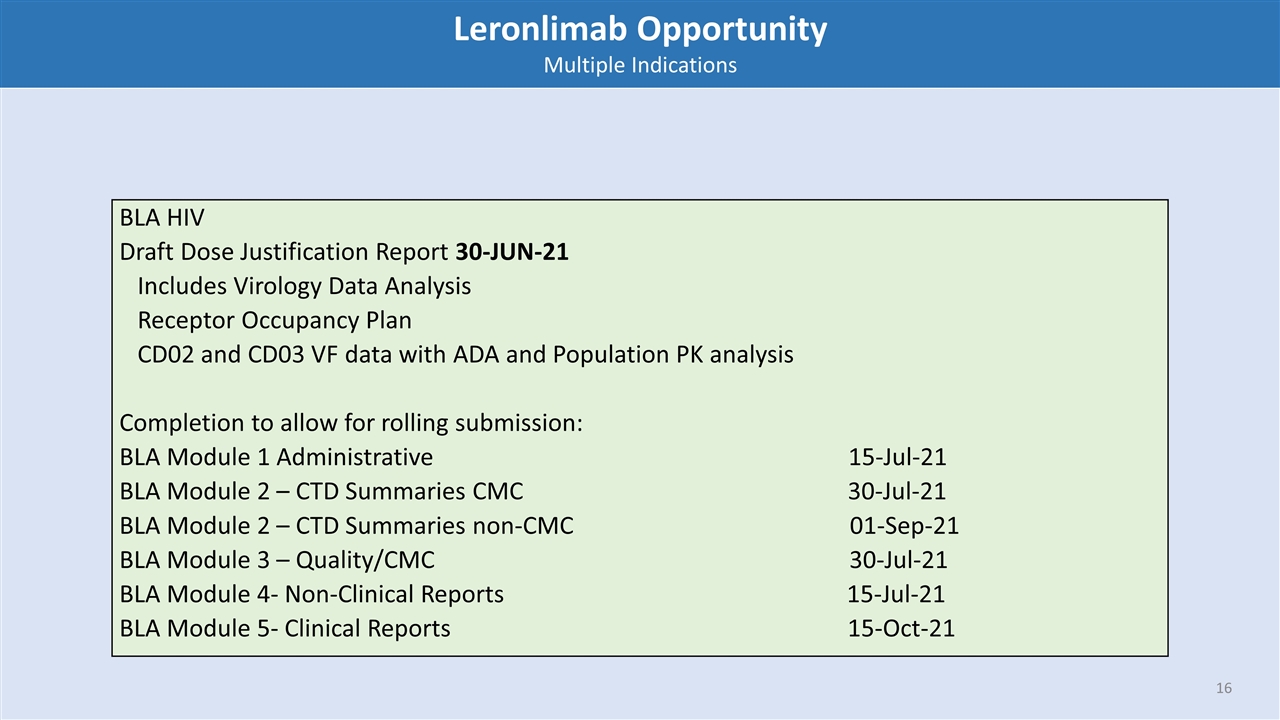
BLA HIV Draft Dose Justification Report 30-JUN-21 Includes Virology Data Analysis Receptor Occupancy Plan CD02 and CD03 VF data with ADA and Population PK analysis Completion to allow for rolling submission: BLA Module 1 Administrative 15-Jul-21 BLA Module 2 – CTD Summaries CMC 30-Jul-21 BLA Module 2 – CTD Summaries non-CMC 01-Sep-21 BLA Module 3 – Quality/CMC 30-Jul-21 BLA Module 4- Non-Clinical Reports 15-Jul-21 BLA Module 5- Clinical Reports 15-Oct-21 Leronlimab Opportunity Multiple Indications

Leronlimab Opportunity Multiple Indications Triple-Negative Breast cancer results in 4-6 months NASH complete enrollment of initial 60 patients end of June NASH enrollment for biomarkers and CT1/PDFF completion Q4 2021 Long-Hauler Phase 3 completion Q4 2021
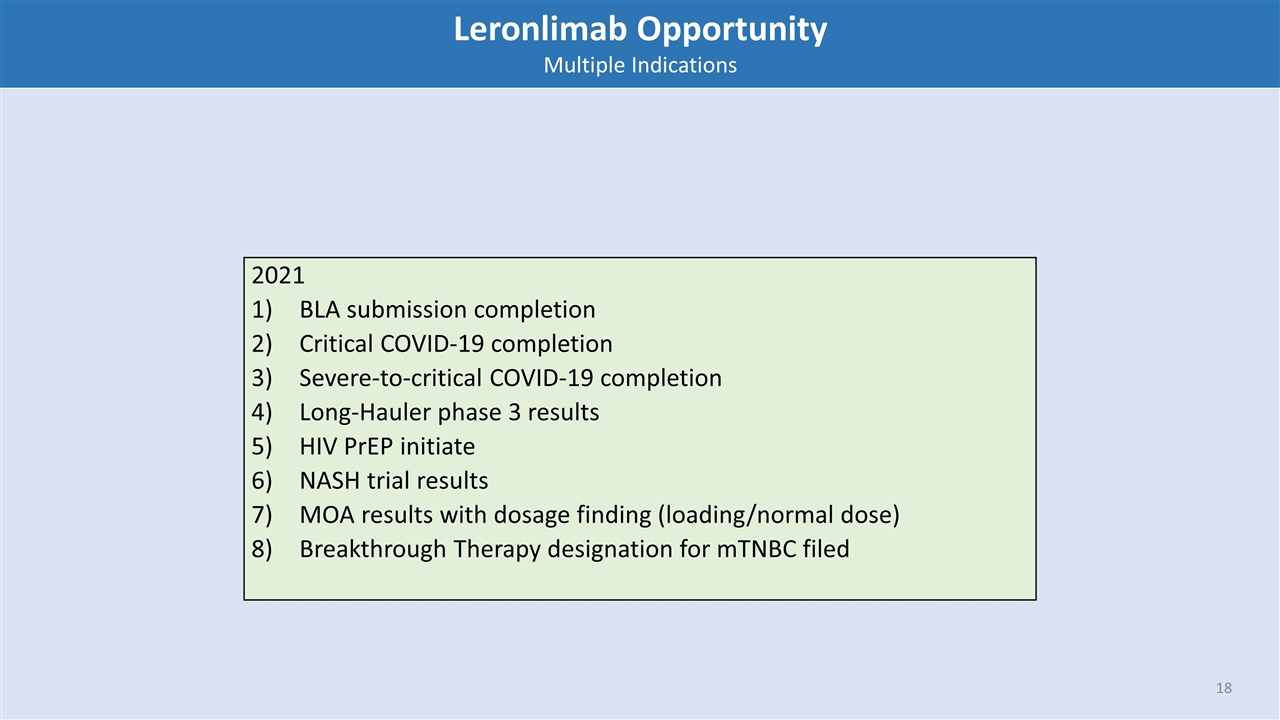
Leronlimab Opportunity Multiple Indications 2021 BLA submission completion Critical COVID-19 completion Severe-to-critical COVID-19 completion Long-Hauler phase 3 results HIV PrEP initiate NASH trial results MOA results with dosage finding (loading/normal dose) Breakthrough Therapy designation for mTNBC filed

Deal signed April 2019 Completion of first batch of clinical grade leronlimab in June 2020 Completion of batches of commercial grade leronlimab in calendar 3Q20-4Q20 Leronlimab Manufacturing Samsung BioLogics (Commercial Partnership) Initial forecast made calendar 1Q20 Updated forecast provided quarterly Order for 2022 and 2023 at new facility at Samsung Contract minimum - 1 million vials/yr Current inventory – 1.1 million vials 2022- 1 million vials minimum 2023 - 2 million vials minimum Order for 2022 and 2023 at new facility at Samsung