

OTCQB: CYDY www.cytodyn.com HIV - Cancer BIOTECH SHOWCASE January 2019 Professor Richard G. Pestell M.D., Ph.D., MB., B.S., F.A.C.P., F.R.A.C.P., F.A.A.A.S., M.B.A. Vice Chairman and Chief Medical Officer Nader Pourhassan, Ph.D. Director, President & CEO ® Leronlimab (PRO 140) ® Trading Symbol CYDY Trading Symbol CYDY Exhibit 99.1

www.cytodyn.com Forward-Looking Statements 2 www.cytodyn.com Trading Symbol: CYDY
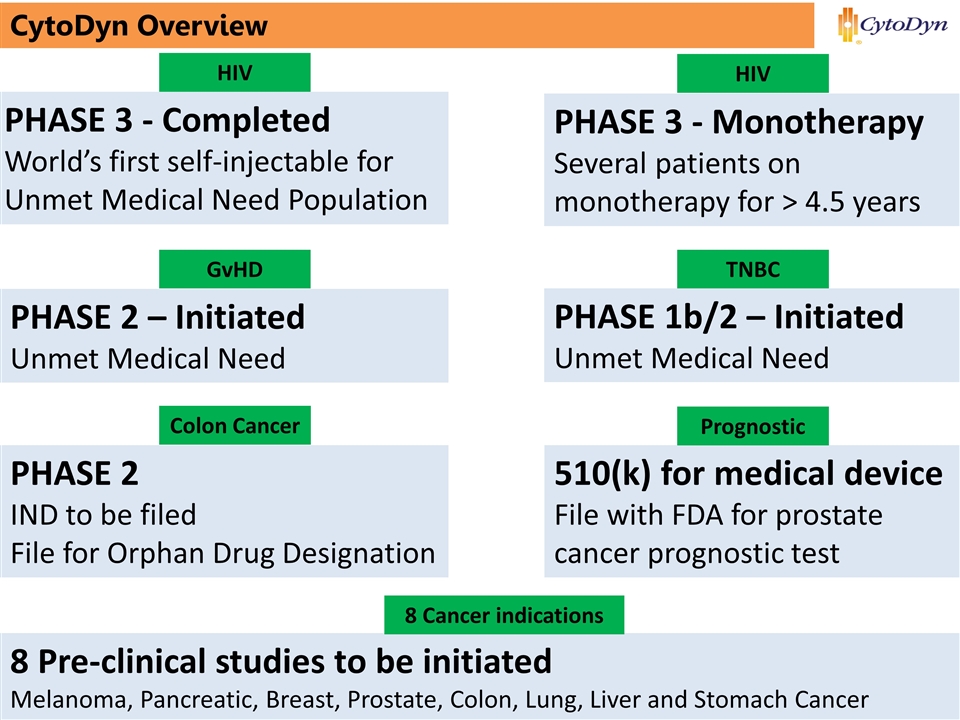
3 www.cytodyn.com CytoDyn Overview PHASE 2 – Initiated Unmet Medical Need PHASE 3 - Completed World’s first self-injectable for Unmet Medical Need Population PHASE 3 - Monotherapy Several patients on monotherapy for > 4.5 years HIV HIV GvHD PHASE 1b/2 – Initiated Unmet Medical Need TNBC PHASE 2 IND to be filed File for Orphan Drug Designation Colon Cancer 510(k) for medical device File with FDA for prostate cancer prognostic test Prognostic Ticker Symbol: CYDY 8 Pre-clinical studies to be initiated Melanoma, Pancreatic, Breast, Prostate, Colon, Lung, Liver and Stomach Cancer 8 Cancer indications
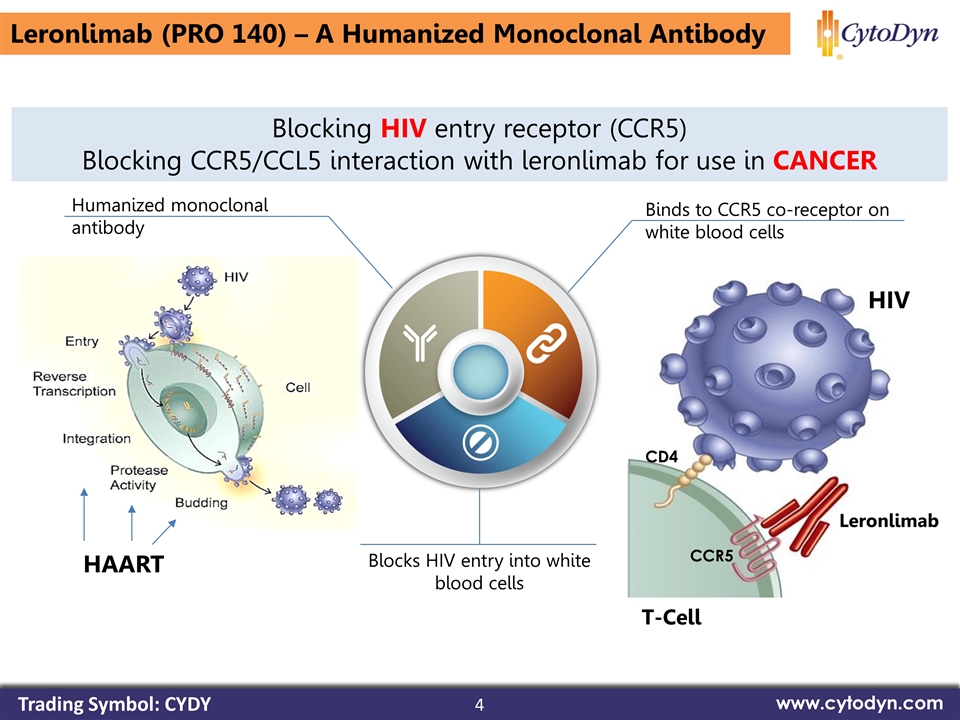
4 www.cytodyn.com Blocking HIV entry receptor (CCR5) Blocking CCR5/CCL5 interaction with leronlimab for use in CANCER Binds to CCR5 co-receptor on white blood cells Blocks HIV entry into white blood cells Leronlimab CCR5 CD4 T-Cell HIV Humanized monoclonal antibody Leronlimab (PRO 140) – A Humanized Monoclonal Antibody HAART Trading Symbol: CYDY
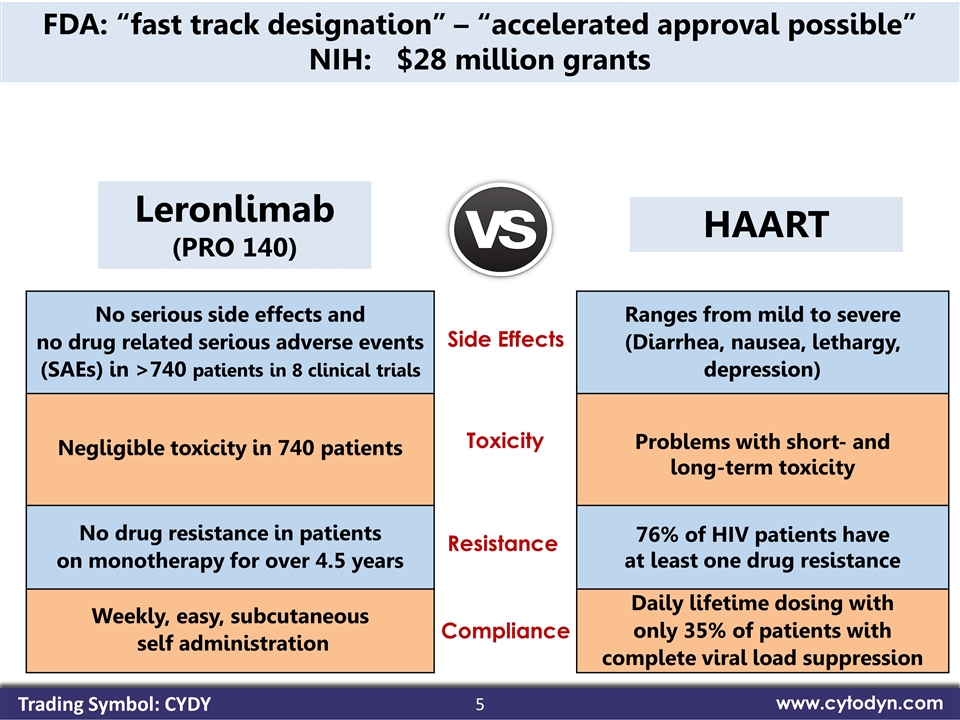
5 www.cytodyn.com Leronlimab (PRO 140) No serious side effects and no drug related serious adverse events (SAEs) in >740 patients in 8 clinical trials Ranges from mild to severe (Diarrhea, nausea, lethargy, depression) Negligible toxicity in 740 patients Problems with short- and long-term toxicity No drug resistance in patients on monotherapy for over 4.5 years 76% of HIV patients have at least one drug resistance Weekly, easy, subcutaneous self administration Daily lifetime dosing with only 35% of patients with complete viral load suppression FDA: “fast track designation” – “accelerated approval possible” NIH: $28 million grants Side Effects Toxicity Resistance Compliance HAART Trading Symbol: CYDY
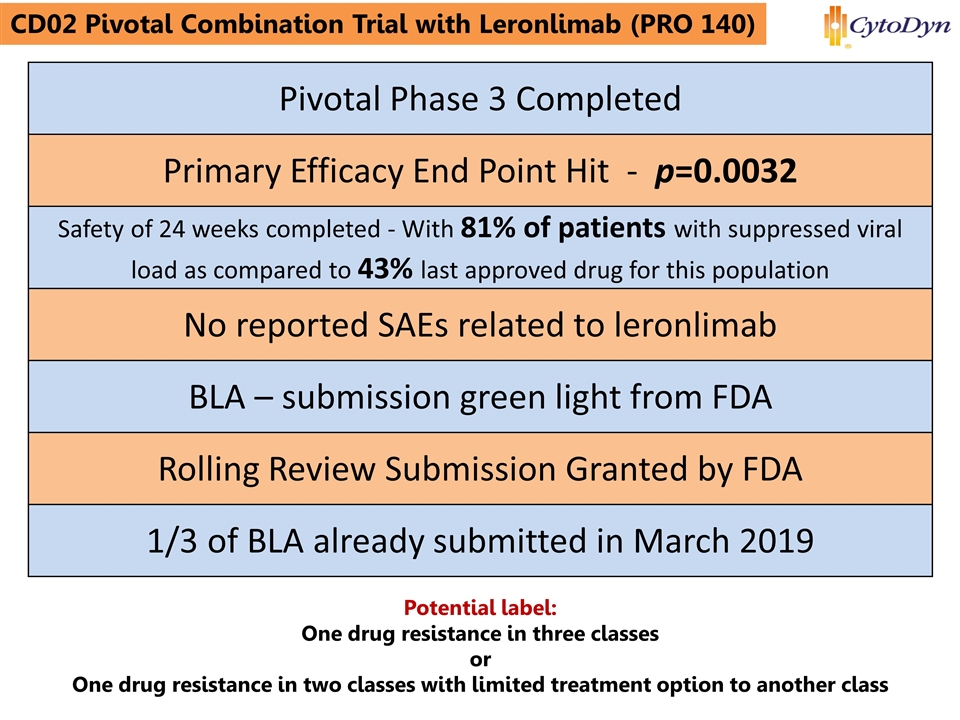
www.cytodyn.com CD02 Pivotal Combination Trial with Leronlimab (PRO 140) Trading Symbol: CYDY Pivotal Phase 3 Completed Primary Efficacy End Point Hit - p=0.0032 Safety of 24 weeks completed - With 81% of patients with suppressed viral load as compared to 43% last approved drug for this population No reported SAEs related to leronlimab BLA – submission green light from FDA Rolling Review Submission Granted by FDA 1/3 of BLA already submitted in March 2019 Potential label: One drug resistance in three classes or One drug resistance in two classes with limited treatment option to another class
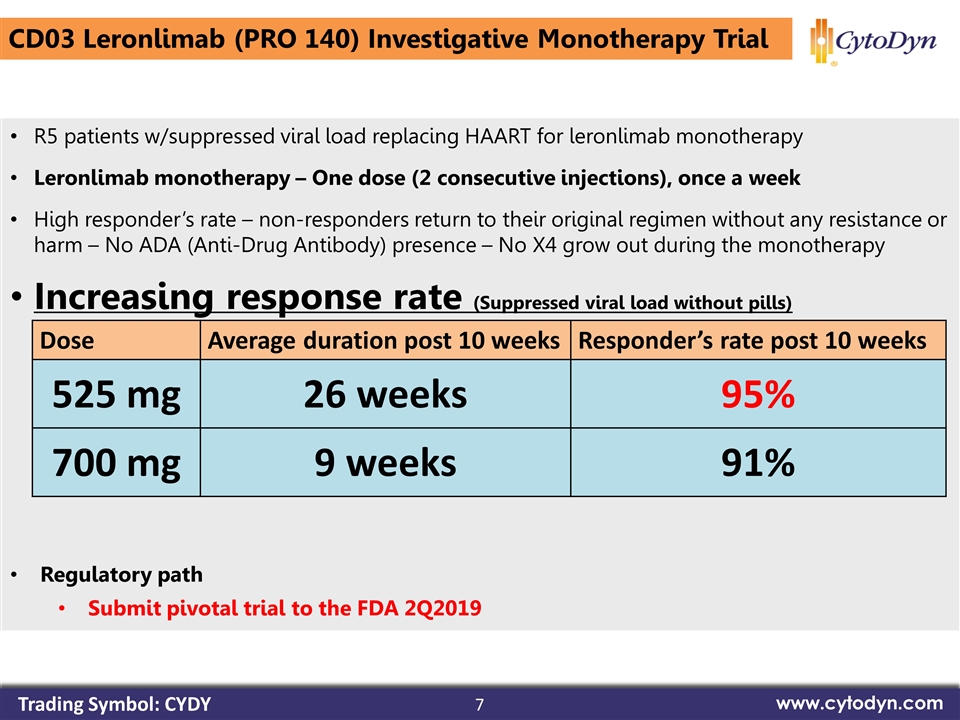
7 www.cytodyn.com CD03 Leronlimab (PRO 140) Investigative Monotherapy Trial R5 patients w/suppressed viral load replacing HAART for leronlimab monotherapy Leronlimab monotherapy – One dose (2 consecutive injections), once a week High responder’s rate – non-responders return to their original regimen without any resistance or harm – No ADA (Anti-Drug Antibody) presence – No X4 grow out during the monotherapy Increasing response rate (Suppressed viral load without pills) Regulatory path Submit pivotal trial to the FDA 2Q2019 Trading Symbol: CYDY Dose Average duration post 10 weeks Responder’s rate post 10 weeks 525 mg 26 weeks 95% 700 mg 9 weeks 91%
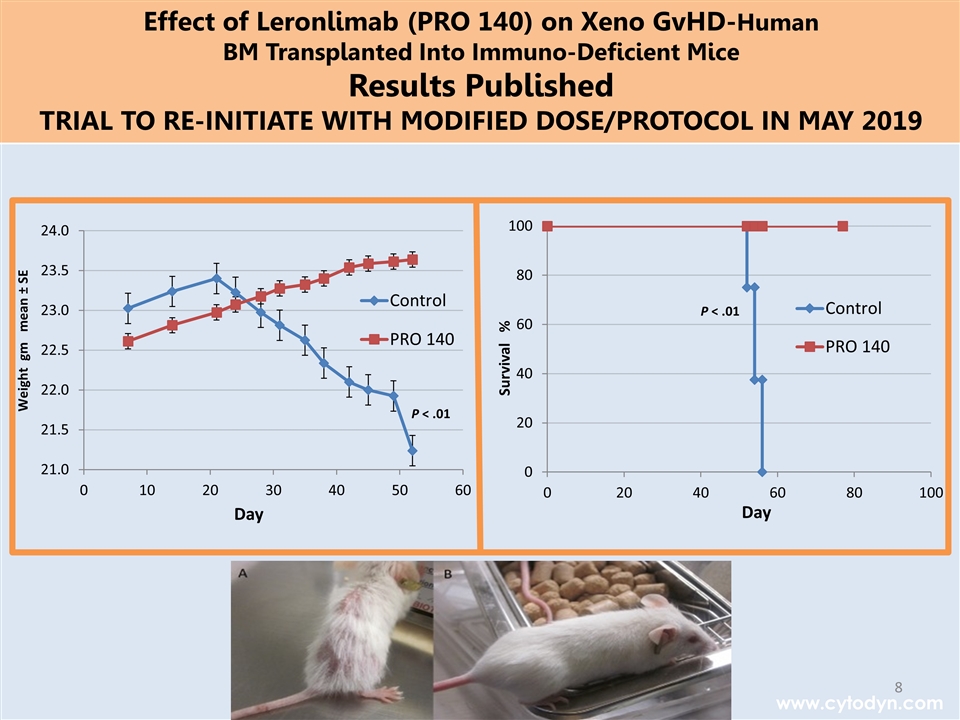
P < .01 P < .01 www.cytodyn.com Effect of Leronlimab (PRO 140) on Xeno GvHD-Human BM Transplanted Into Immuno-Deficient Mice Results Published TRIAL TO RE-INITIATE WITH MODIFIED DOSE/PROTOCOL IN MAY 2019
Expansion into Cancer Indications 9 www.cytodyn.com Named world-renowned oncologist Dr. Richard Pestell Chief Medical Officer and Vice Chairman (https://www.youtube.com/watch?v=98J1HgCm8wU) Leads leronlimab (PRO 140) non-HIV development programs Led 2 National Cancer Institute-designated cancer centers Lombardi Comprehensive Cancer Center at Georgetown University Sidney Kimmel Cancer Center at Thomas Jefferson University Executive Vice President Thomas Jefferson University (25,000 employees, $5.6B) Founded ProstaGene to develop CCR5 technology in cancer Issued patents for technology on metastasis (many types of cancer) Showed > 50% of 2,200 patients -increased CCR5 in breast cancer CCR5 inhibitors blocked breast, prostate and colon cancer metastasis in pre-clinical studies Trading Symbol: CYDY
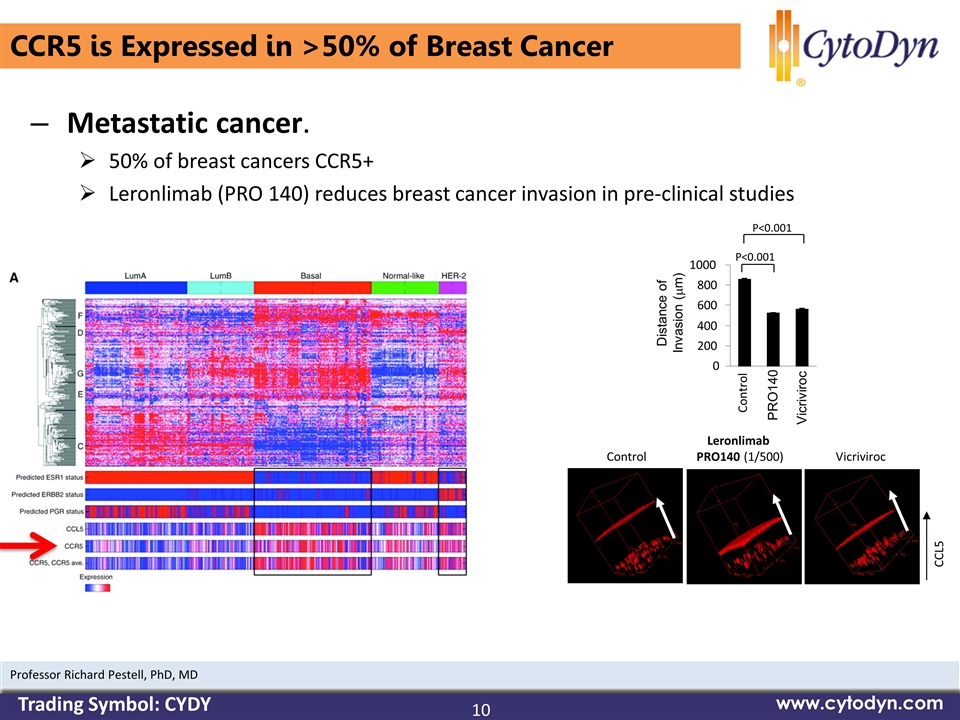
CCR5 is Expressed in >50% of Breast Cancer Metastatic cancer. 50% of breast cancers CCR5+ Leronlimab (PRO 140) reduces breast cancer invasion in pre-clinical studies 0 200 400 600 800 1000 Control PRO140 Vicriviroc Distance of Invasion (mm) P<0.001 P<0.001 Control Leronlimab PRO140 (1/500) Vicriviroc CCL5 10 www.cytodyn.com Professor Richard Pestell, PhD, MD Trading Symbol: CYDY
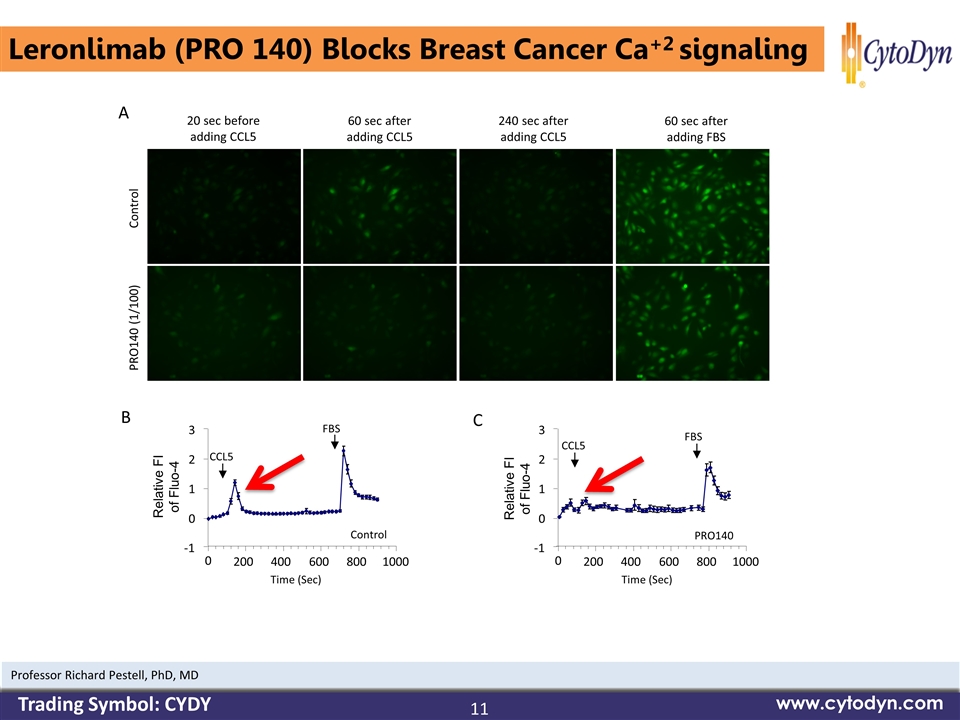
Control PRO140 (1/100) 20 sec before adding CCL5 60 sec after adding CCL5 240 sec after adding CCL5 60 sec after adding FBS 0 200 400 600 800 1000 CCL5 FBS 0 200 400 600 800 1000 CCL5 FBS Control PRO140 A B C Time (Sec) Time (Sec) -1 0 1 2 3 -1 0 1 2 3 Relative FI of Fluo-4 Relative FI of Fluo-4 Leronlimab (PRO 140) Blocks Breast Cancer Ca+2 signaling 11 www.cytodyn.com Professor Richard Pestell, PhD, MD Trading Symbol: CYDY
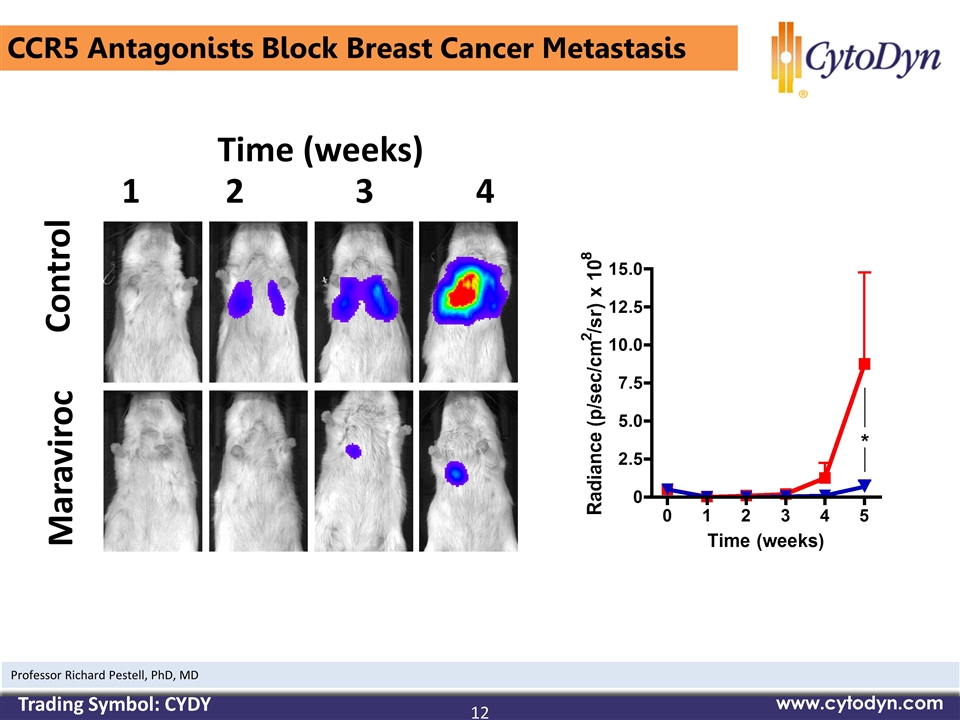
CCR5 Antagonists Block Breast Cancer Metastasis Time (weeks) 1 2 3 4 Control Maraviroc 12 www.cytodyn.com Professor Richard Pestell, PhD, MD Trading Symbol: CYDY
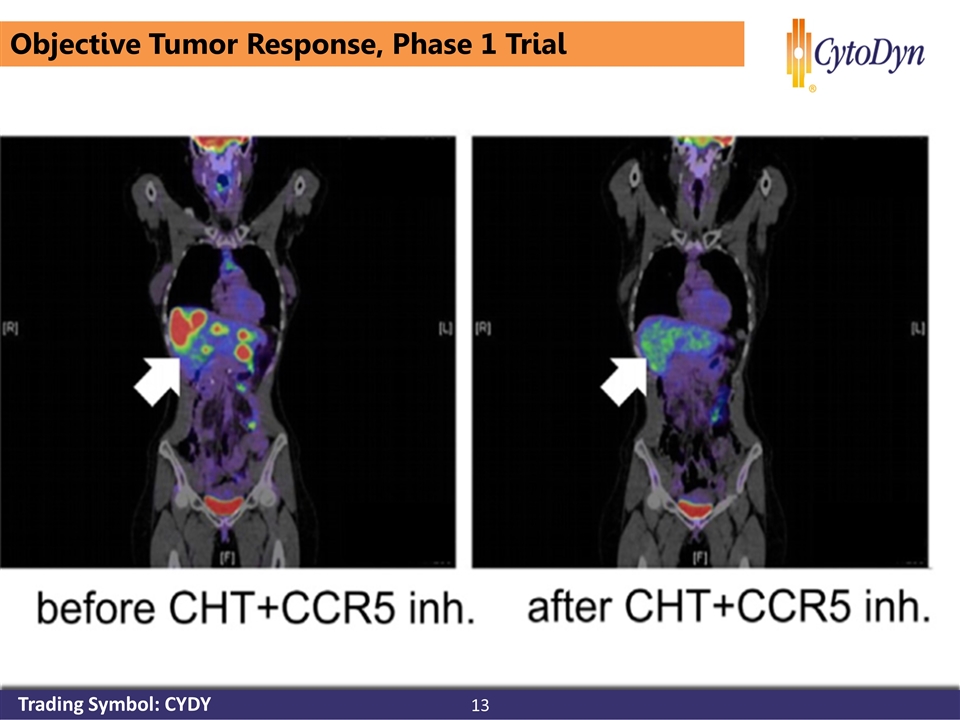
advanced-stage metastatic colorectal cancer who are refractory to standard chemotherapy, including regorafenib Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients Cancer Cell. 2016 587-601 Objective Tumor Response, Phase 1 Trial 13 Trading Symbol: CYDY
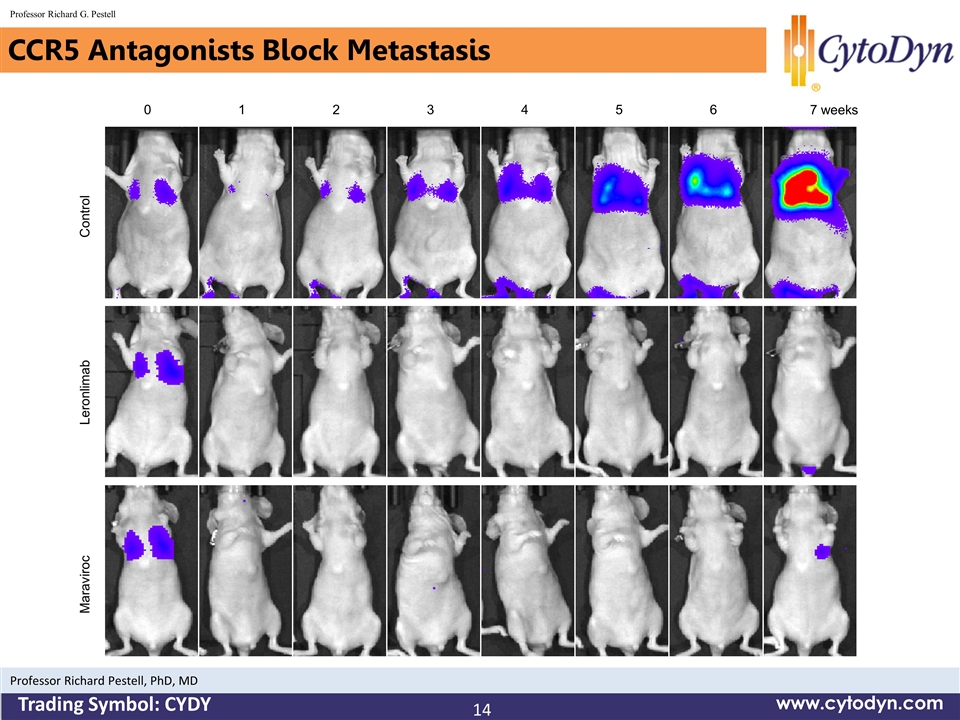
CCR5 Antagonists Block Metastasis 14 www.cytodyn.com Professor Richard G. Pestell Professor Richard Pestell, PhD, MD Trading Symbol: CYDY 0 1 2 3 6 5 7 weeks 4 Control Leronlimab Maraviroc
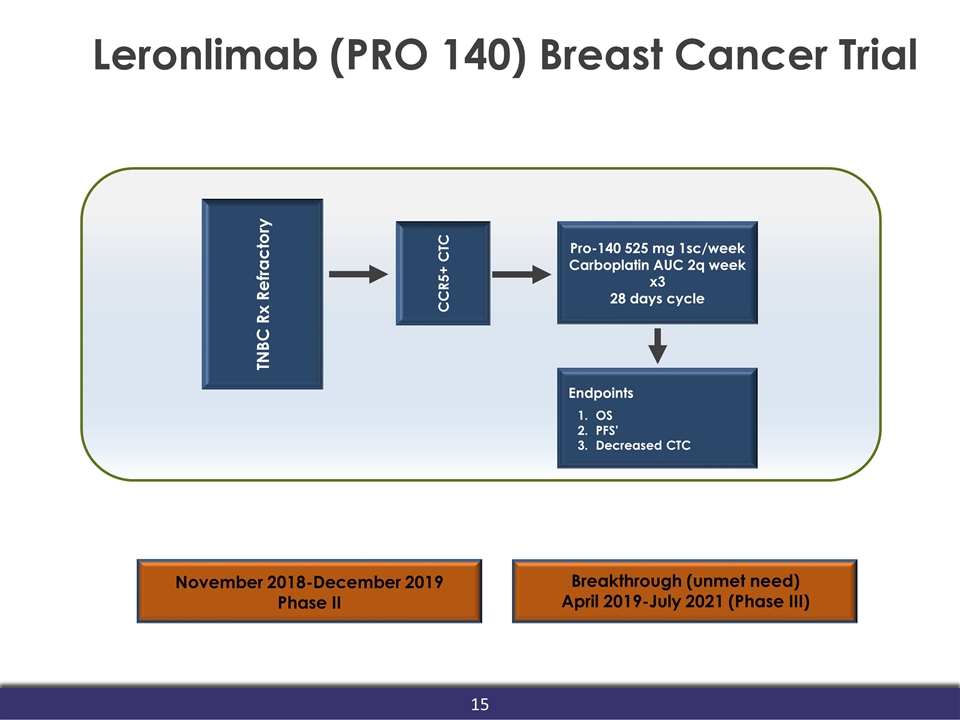
Leronlimab (PRO 140) Breast Cancer Trial November 2018-December 2019 Phase II Breakthrough (unmet need) April 2019-July 2021 (Phase III) Pro-140 525 mg 1sc/week Carboplatin AUC 2q week x3 28 days cycle TNBC Rx Refractory CCR5+ CTC Endpoints OS PFS’ Decreased CTC 15
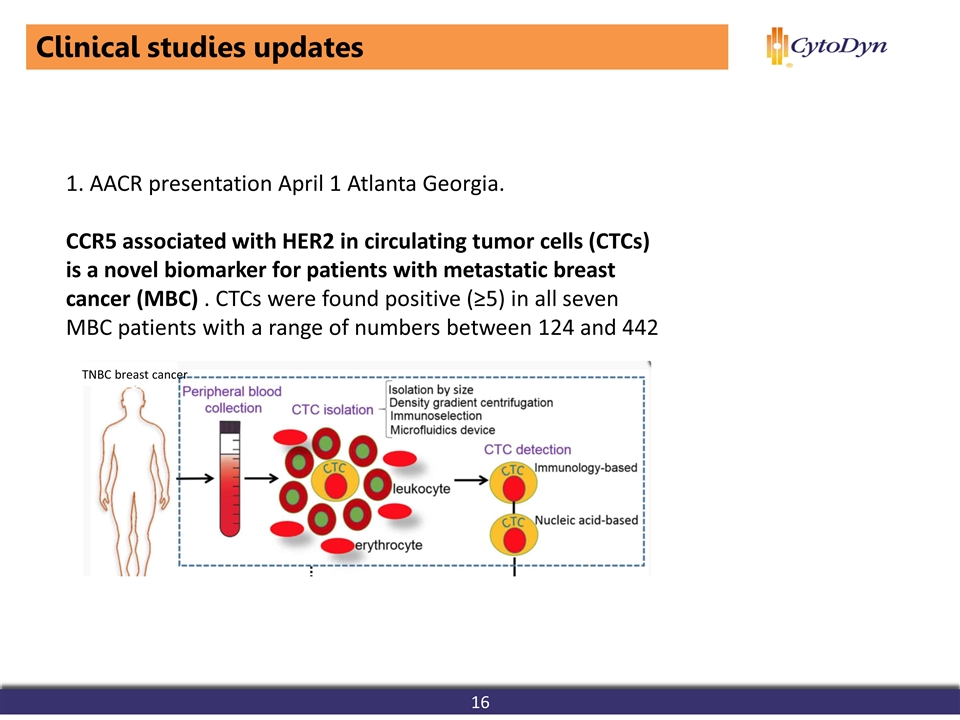
1. AACR presentation April 1 Atlanta Georgia. CCR5 associated with HER2 in circulating tumor cells (CTCs) is a novel biomarker for patients with metastatic breast cancer (MBC) . CTCs were found positive (≥5) in all seven MBC patients with a range of numbers between 124 and 442 Clinical studies updates 16 TNBC breast cancer
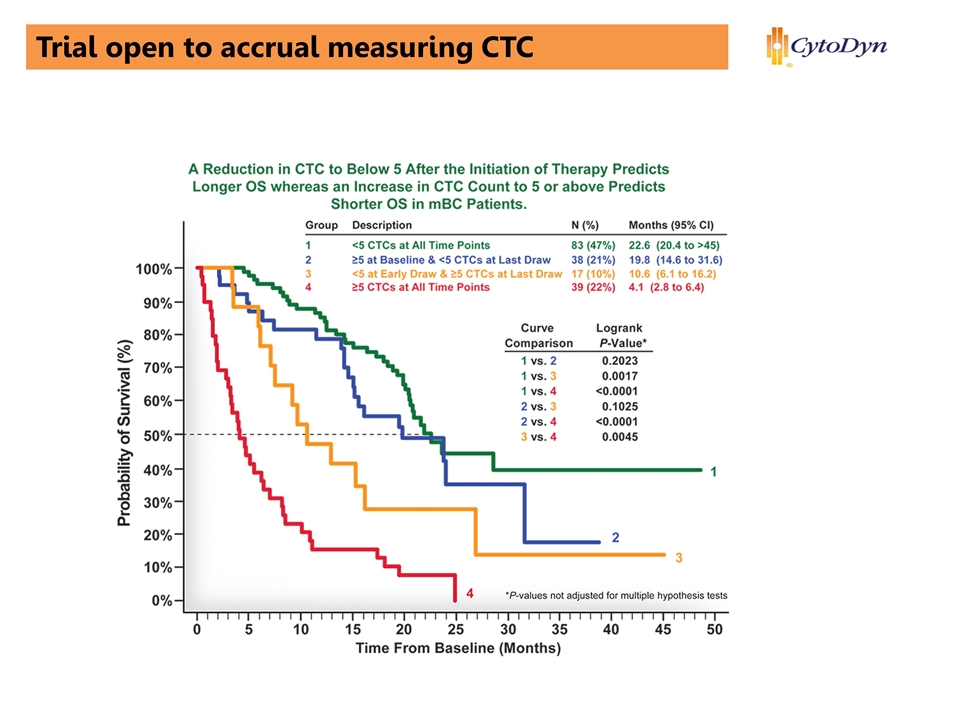
Trial open to accrual measuring CTC
1. Pacific Hematology Oncology Associates Dr. Milana Dolezal mdolezal@phoamd.com 2100 Webster street suite 220, San Francisco, ca 9411 david@PHOAMD.COM 415-923-3012 Other sites to open: Northwestern University Medical School, Methodist Houston, Vanderbilt University, Sidney Kimmel Cancer Center. Trial open to accrual * * * * *
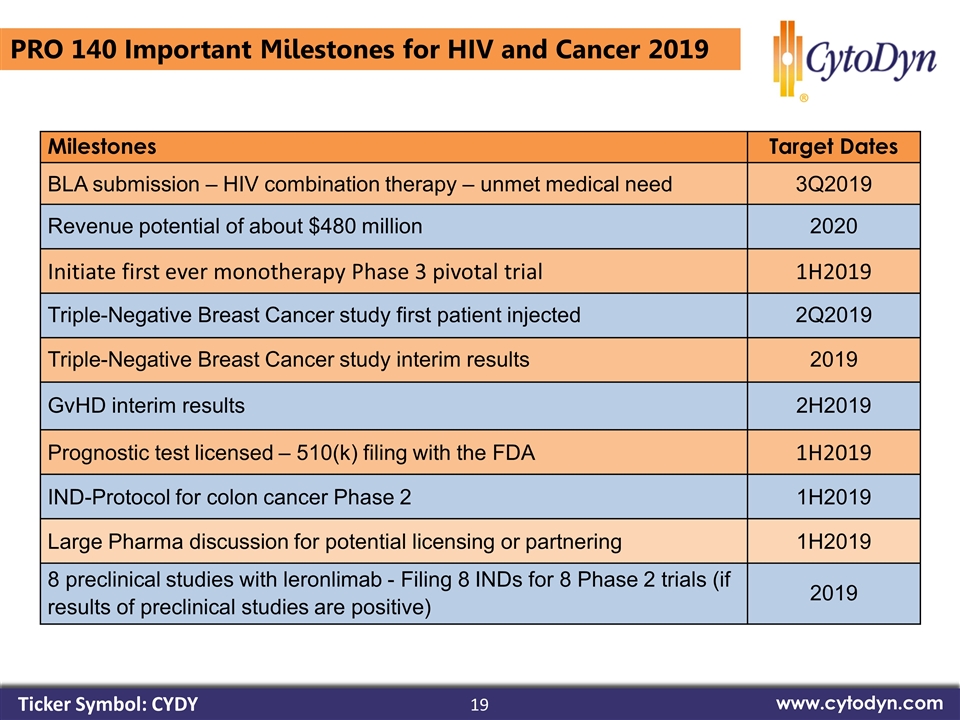
19 www.cytodyn.com Milestones Target Dates BLA submission – HIV combination therapy – unmet medical need 3Q2019 Revenue potential of about $480 million 2020 Initiate first ever monotherapy Phase 3 pivotal trial 1H2019 Triple-Negative Breast Cancer study first patient injected 2Q2019 Triple-Negative Breast Cancer study interim results 2019 GvHD interim results 2H2019 Prognostic test licensed – 510(k) filing with the FDA 1H2019 IND-Protocol for colon cancer Phase 2 1H2019 Large Pharma discussion for potential licensing or partnering 1H2019 8 preclinical studies with leronlimab - Filing 8 INDs for 8 Phase 2 trials (if results of preclinical studies are positive) 2019 PRO 140 Important Milestones for HIV and Cancer 2019 Ticker Symbol: CYDY

OTCQB: CYDY www.cytodyn.com HIV - Cancer BIOTECH SHOWCASE January 2019 Professor Richard G. Pestell M.D., Ph.D., MB., B.S., F.A.C.P., F.R.A.C.P., F.A.A.A.S., M.B.A. Vice Chairman and Chief Medical Officer Nader Pourhassan, Ph.D. Director, President & CEO ® Leronlimab (PRO 140) ® Trading Symbol CYDY Trading Symbol CYDY